> Abstract
Τhyroid neoplasia accounts for 1-3,8% of all canine tumours, and the most common types include adenoma and carcinoma, the latter being identified in 90% of cases. Exposure to radiation, iodine deficiency, hypothyroidism, or even genetic mutations, are included among the triggering factors. Benign adenomas are usually small and movable, without infiltration of surrounding tissues and the presence of metastasis. In contrast, carcinomas are larger, with extensive infiltrating tendencies, primarily in the lungs and regional lymph nodes. Diagnosis is based on clinical signs emerging due to compression of adjacent organs (oesophagus, trachea, larynx, pharynx), endocrine testing and diagnostic imaging. To reach a definitive diagnosis biopsies for histopathological examination need to be obtained, which will confirm the origin of the mass and will differentiate benign from malignant neoplastic tissue. In cases of thyroid adenoma, surgical excision of the tumour is the treatment of choice and leads to clinical cure. The treatment of choice and the prognosis for thyroid carcinoma depend on tumour size, the extent of infiltration of surrounding tissues, the presence of metastases and the possibility of using alternative treatments. Surgical management is indicated in cases of small and movable carcinomas or with superficial infiltration of surrounding tissues, but it is not recommended in cases of extensively infiltrative and fixed carcinomas.
> Introduction
Τhyroid tumours represent 1-3,8 % of all canine tumours while 10% and 15% of neoplasms affecting the head and cervical region, respectively.1-5 Thyroid tumour types include adenoma and carcinoma, the latter being identified more often in 90 % of cases, and in dogs of median age ranging between 9 and 11 years.1,3,6-10 Beagles, Siberian Huskies, Boxers and Golden Retrievers are predisposed to thyroid neoplasia,1,3,4,11,12 but gender predisposition has not been noted.1-3,9-14
> Initiating causes and predisposing factors
Previously published studies in people as well as dogs have proven that thyroid exposure to radiation and iodine deficiency have been implicated in neoplastic growth in the gland.15 Iodine deficiency causes over secretion of thyroid-stimulating hormone (TSH) from the pituitary gland, which leads to thyroid epithelial cell hyperplasia and possibly to metaplasia.16,17 Furthermore, it has been proven that hypothyroidism caused by lymphocytic thyroiditis is related to thyroid neoplastic transformation in dogs.17 Studies from human medicine confirm that various mutations in the ras oncogenes and tumour suppressor genes can trigger thyroid neoplastic transformation and benign neoplasms may undergo malignant transformation respectively.18,19 Finally, aneuploidy is a common characteristic of canine thyroid carcinoma and it is identified at a rate higher than 50% of cases with primary tumours.20
> Biological behaviour
Thyroid tumours are usually located in the area surrounding the gland, including the ventral or ventrolateral surface of the cervical region, caudal to the larynx.3,10 The right and left lobe are affected with the same frequency, whereas 36-40% of carcinomas affect both lobes.1,7,8,11 Rarely neoplasms can be observed in ectopic sites, such as the base of the tongue, or they can be sublingual, and found subcutaneously dorsal to the trachea,21 in the cranial mediastinum, at the heart base, and in other endocardial sites.22-26 Benign adenomas develop at a slow rate, they are usually small, ranging from microscopical to several centimeters in diameter, soft, compact, well encapsulated and movable along their craniocaudal axis in the subcutaneous cervical region. They do not infiltrate surrounding tissues, they do not metastasise and they are usually discovered as an incidental finding during necropsy.1,6,11
Malignant carcinomas usually originate from follicular (adenocarcinoma) and rarely from parafollicular or C cells (myeloid carcinoma).5,27,28 They grow fast and are usually nodular, larger than adenomas, ranging in size from microscopical to a diameter over 10 centimeters.1,3,29 Their biological behaviour is extensively infiltrative affecting adjacent tissue structures such as the larynx, the trachea, the jugular veins, and the carotid sheath and they have a predilection for regional or distal metastasis.1,3,6 Τhyroid carcinomas usually metastasise in the lungs and regional lymph nodes, and rarely in the jugular vein, the heart, the kidneys, the adrenal glands, the liver, the spleen, the intestine, the omentum, and the brain.3,11 The possibility of diagnosing metastatic lesions at the time of diagnosis ranges from 16 to 38% and is directly related to tumour size.3,6,7,10 In a large retrospective study it was noted that all dogs in which the size of the carcinoma exceeded 100 cm3, would have distal metastatic sites in 100%, whereas in cases of carcinoma ranging from 21 to 100 cm3 and less than 20 cm3 metastatic lesions would be identified in 74% and 14% of cases, respectively.11
> Diagnosis and Staging
Clinical signs
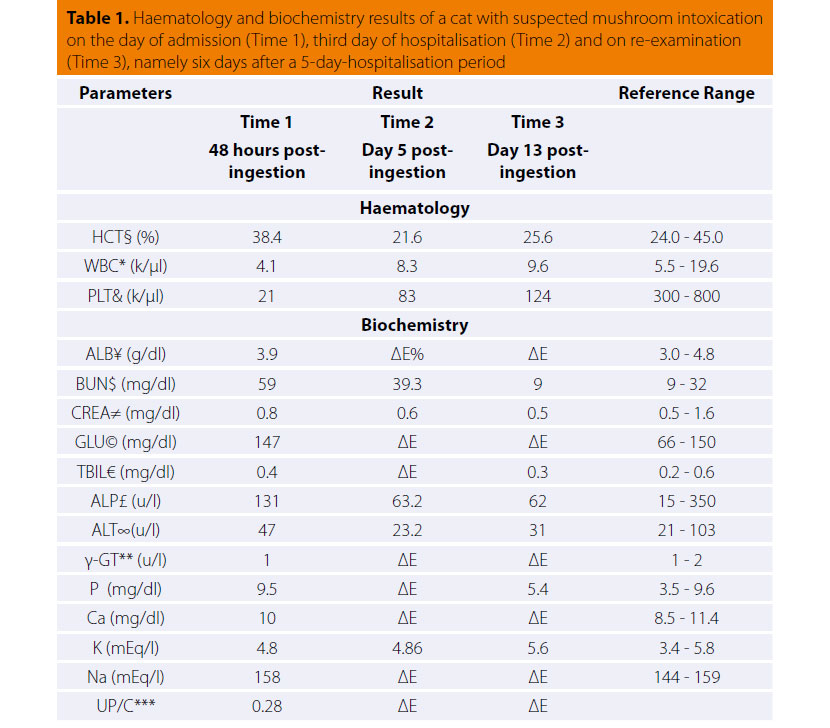
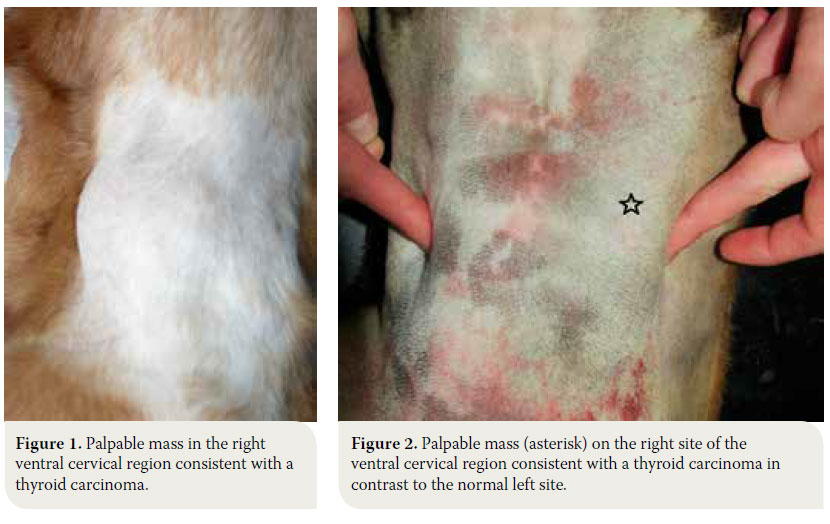
The dog is usually admitted after the owner has noted a mass in the cervical region, which rarely extends toward the thoracic inlet (Figures 1, 2).1-3,7,10,11 This mass is usually noted ventral to the trachea (65- 70 of cases %), is related to the larynx and can be well defined or invasive.11 Information from the history and physical examination can identify clinical sings from the respiratory and gastrointestinal tract. Τhe large size of the neoplasm, metastatic lesions in the lungs and lymph nodes and compression of adjacent tissues (oesophagus, trachea, larynx, pharynx) can manifest with signs including respiratory distress, coughing, wheezing, cyanosis, sneezing, reverse sneezing, laryngeal paralysis, dysphonia, regurgitation, vomiting, lethargy, peripheral lymphadenopathy, fever and Horner’s syndrome.1-3,11,30
It is worthy of note that 60% of dogs will remain clinically euthyroid, whereas 30% present with signs of hypothyroidism due to obliteration of the normal gland parenchyma.1-3,11 Cases of functional neoplasm have been reported in 10-22% of cases, when dogs developed signs of hyperthyroidism similar to hyperthyroid cats, including polyuria, polydipsia, polyphagia with simultaneous weight loss, generalised muscle weakness, restlessness, exercise intolerance, tachycardia and continuous searching for cold areas in the home environment due to a low tolerance to heat.3,29,31
Endocrine testing
Considering that most dogs are admitted in a euthyroid state, there are very few clinical studies evaluating thyroid function via estimation of hormone levels including triiodothyronine (Τ3), thyroxine (Τ4) and anterior pituitary lobe TSH.3,11 From a study in 36 dogs with thyroid tumours, 39% had reduced levels of Τ3 and Τ4, none of them presenting with clinical signs of hypothyroidism, whereas 31% had increased levels of Τ4, with only 2 cases presenting with clinical signs of hyperthyroidism.9 Irrespective of clinical signs of hypothyroidism or hyperthyroidism, it is recommended that all cases have total Τ4, free Τ4 and TSH levels measured to evaluate thyroid function.32
Diagnostic imaging
Radiography
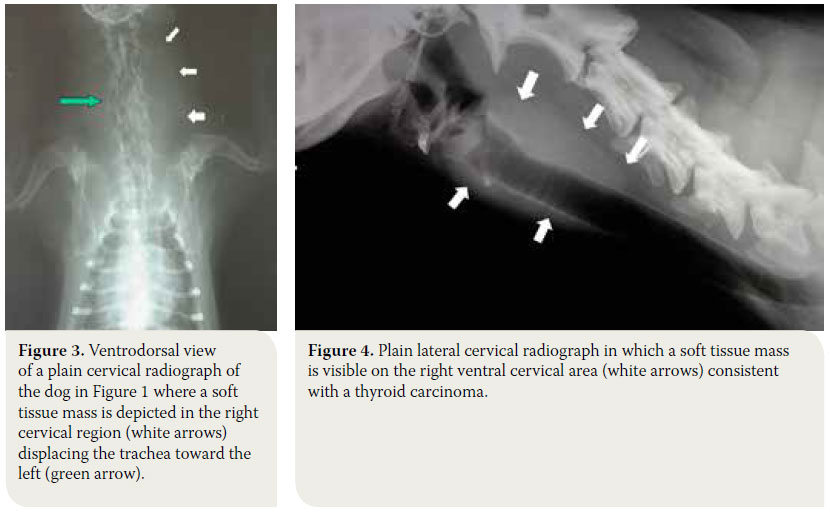
Radiographs of the cervical region will reveal, almost in every case, the presence of a soft tissue mass that may or may not displace the trachea or larynx, however, it does not provide information regarding vascular support and infiltration of surrounding tissues (Figures 3, 4).6 Thoracic radiography reveals the presence of pulmonary metastatic lesions in 33-77% of cases.1-3,11 If mediastinal findings compatible with a soft tissue mass that may related to an ectopic thyroid tumor are noted, ultrasonographic evaluation of the thorax is indicated, because the presence of fluid or fat in the thoracic cavity or the mediastinum may resemble a mass in plain radiographs.30,33
Ultrasonography
This is a valuable, relatively inexpensive non-invasive imaging modality, for which anaesthesia is rarely required. For all the previous reasons, it is often used as the method of choice to evaluate thyroid morphology. Ultrasonography can discern masses >2mm and can differentiate between solid and cystic nodules. Consequently, ultrasonographic evaluation is recommended for lesions <2 cm.34 The information that ultrasonography provides aids in differentiating thyroid tumours from other mass lesions that can be found in the ventral aspect of the cervical region and can determine if the gland is unilaterally or bilaterally affected.35 However, it can sometimes be challenging to substantiate if the mass originated from the thyroid gland if the normal anatomy of the cervical region has been altered.33,36 Moreover, it provides information about the degree of tumour vascularization and surrounding tissue infiltration, however without differentiating between benign and malignant neoplasms, unless peripheral lymphadenopathy and local infiltration are present.33 In case of tumours with an extensive vascular supply, ultrasound-guided needle aspiration biopsy is recommended to avoid any haemorrhage.30,33
 Computed tomography (CT) and magnetic resonance imaging (MRI)
Computed tomography (CT) and magnetic resonance imaging (MRI)
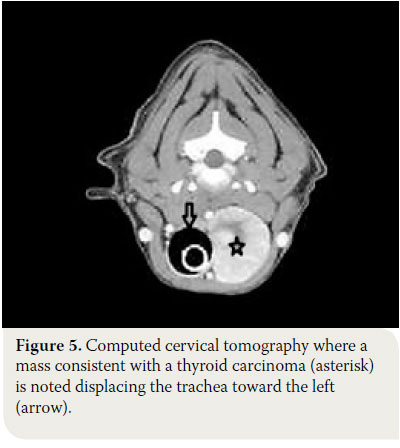
These imaging modalities are indicated for masses >3cm and can provide useful information regarding the origin and the degree of vascularisation, as well as the extent of surrounding tissue infiltration.30,34 Moreover, they offer the possibility of investigating the thorax for metastatic pulmonary lesions and the presence of ectopic intrathoracic thyroid tissue, and they can even detect metastasis in the lymph nodes.34 Finally, CT aids in the staging of thyroid carcinoma. 37
In conclusion, all 3 imaging modalities (ultrasonography, CT or MRI) provide almost the same information regarding the presence of a cervical mass. In a study that compared sensitivity and specificity between these techniques in 13 cases of canine thyroid carcinoma, it was found that ultrasonography had 79% sensitivity and 33% specificity, CT 85% and 100% and MRI 93% and 67% respectively.36 In cases when interpretation of diagnostic imaging results are controversial, surgical exploration of the cervical region is a fast, accurate and inexpensive way to identify the degree of invasiveness and the origins of the mass.38
Cytological examination
Fine needle aspiration of the mass (FNA) is a noninvasive, fast and easy procedure, which does not necessitate sedation or general anaesthesia.38 Studies have shown that FNA cytology can identify a cervical mass of thyroid origin in more than half of the cases with canine thyroid neoplasia, without being able to differentiate between benign and malignant tumours.3,29 Blood contamination of the samples due to extensive neoplastic tissue vascularisation, the fragility of thyroid follicular cells and the presence of active inflammation, are some of the reasons that limit the diagnostic accuracy of this technique.29 In order to minimise sample contamination with blood, it is recommended that fine needle aspiration should be ultrasound guided.30,33
Biopsy and histopathology
In small (<7cm) and/or movable masses, excisional biopsy can be diagnostic as well as therapeutic. Biopsy from large and severely invasive tumours can be done with a Tru-Cut needle or excision although there is a risk of profuse haemorrhage.6,39 Therefore, open surgical biopsy is recommended so that samples can be obtained from a rather avascular region and bleeding can be controlled if necessary.39
In order to reach the definitive diagnosis of thyroid tumours, histopathology of biopsy samples is mandatory, which not only confirms tumour origin but can also differentiate between benign and malignant tumours.29 Based on histology, thyroid tumours originate either from thyroid follicular cells or from parafollicular or C-cells.
Malignancy in well differentiated tumours is characterised by extracapsular extension and vascular infiltration. 40 Most canine thyroid carcinomas are well to moderately differentiated and usually originate from thyroid follicular cells. The mixed solid-cystic form is most commonly encountered, followed by the cystic and the solid form.3,7,24 Follicular adenomas usually belong to the follicular type.11,41 The papillary type of follicular carcinoma, medullary carcinoma (parafollicular or C-cells) and undifferentiated carcinomas rarely occur in dogs.1-3,11,28 Survival time for dogs with thyroid carcinoma does not seem to be significantly correlated to histopathological stage.3,7
Staging
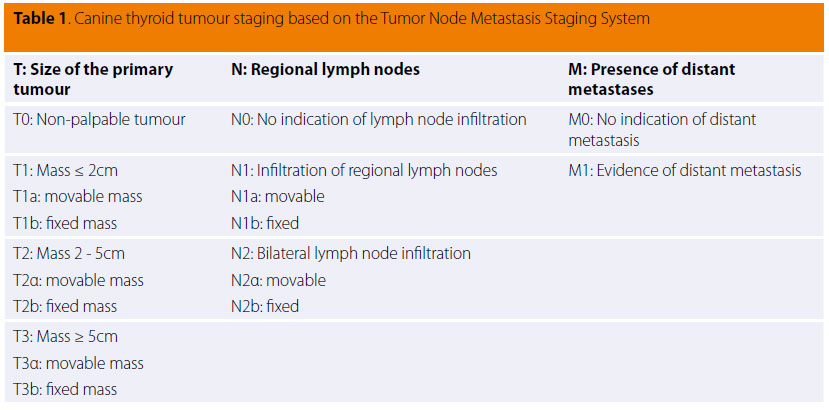
Clinical staging is determined according to the World Health Organisation (WHO TNM Staging System) and is summarised in Table 1. According to this system, the tumour is categorised based on size and surrounding tissue infiltration (Τ), metastasis in regional lymph nodes (N) or the presence of distant metastasis (Μ).42
> Differential diagnosis
A thyroid neoplasm should be differentiated from abscess, granuloma, salivary mucocele, lymphogenic metastasis of tonsilar squamous cell carcinoma, lymphoma, carotid body tumour and sarcoma.43
> Treatment
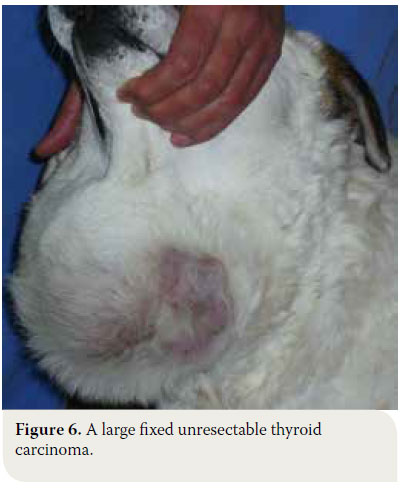 In cases of thyroid adenoma, surgical excision of neoplastic tissue is the treatment of choice and leads to clinical cure.6,32 Regarding thyroid carcinoma, treatment options depend on factors including tumour size, the extent of surrounding tissue infiltration, the presence of metastatic lesions and the possibility of using alternative treatment modalities (radiation therapy, chemotherapy).43 In general, surgical treatment is indicated in cases of small (<7cm) and movable neoplasms or tumours with superficial infiltration of surrounding tissues, but it is contraindicated in cases of severely invasive and fixed tumours (Figure 6).6,7,10,32 In many cases, surgical exploration of the carcinoma is necessary in order to identify the extent of surrounding tissue invasion. In cases where metastatic lesions are present at the time of diagnosis, treatment is palliative and includes monotherapy or a combination of surgical excision and radiation therapy. In cases when diagnostic imaging has not demonstrated the presence of metastasis, selection of treatment is based on whether the tumour is movable or fixed. If it is movable, surgical excision by unilateral or more rarely bilateral thyroidectomy is undertaken, combined or not with radiotherapy. When the tumour is fixed, surgical treatment is contraindicated and radiotherapy and radioactive iodine administration are recommended.32
In cases of thyroid adenoma, surgical excision of neoplastic tissue is the treatment of choice and leads to clinical cure.6,32 Regarding thyroid carcinoma, treatment options depend on factors including tumour size, the extent of surrounding tissue infiltration, the presence of metastatic lesions and the possibility of using alternative treatment modalities (radiation therapy, chemotherapy).43 In general, surgical treatment is indicated in cases of small (<7cm) and movable neoplasms or tumours with superficial infiltration of surrounding tissues, but it is contraindicated in cases of severely invasive and fixed tumours (Figure 6).6,7,10,32 In many cases, surgical exploration of the carcinoma is necessary in order to identify the extent of surrounding tissue invasion. In cases where metastatic lesions are present at the time of diagnosis, treatment is palliative and includes monotherapy or a combination of surgical excision and radiation therapy. In cases when diagnostic imaging has not demonstrated the presence of metastasis, selection of treatment is based on whether the tumour is movable or fixed. If it is movable, surgical excision by unilateral or more rarely bilateral thyroidectomy is undertaken, combined or not with radiotherapy. When the tumour is fixed, surgical treatment is contraindicated and radiotherapy and radioactive iodine administration are recommended.32
Surgical treatment
Surgical anatomy
Sound knowledge of the anatomy of the cervical region and of the thyroid gland in particular is necessary for the unimpeded and safe surgical exploration and management of thyroid tumours. The thyroid gland is formed by two elongated lobes and is located at the level of the cranial aspect of the cervical trachea. Usually the right lobe is located at the level of the first five tracheal rings, whereas the left lobe is located more caudally, extending from the third to the eighth tracheal ring. The lobes come in contact ventrally with the sternohyoid and sternocephalicus muscles, and laterally with the sternothyroid muscle. The lobes are located close to the carotid artery, the cervical sympathetic trunk and the jugular vein (Figure 7).44-45
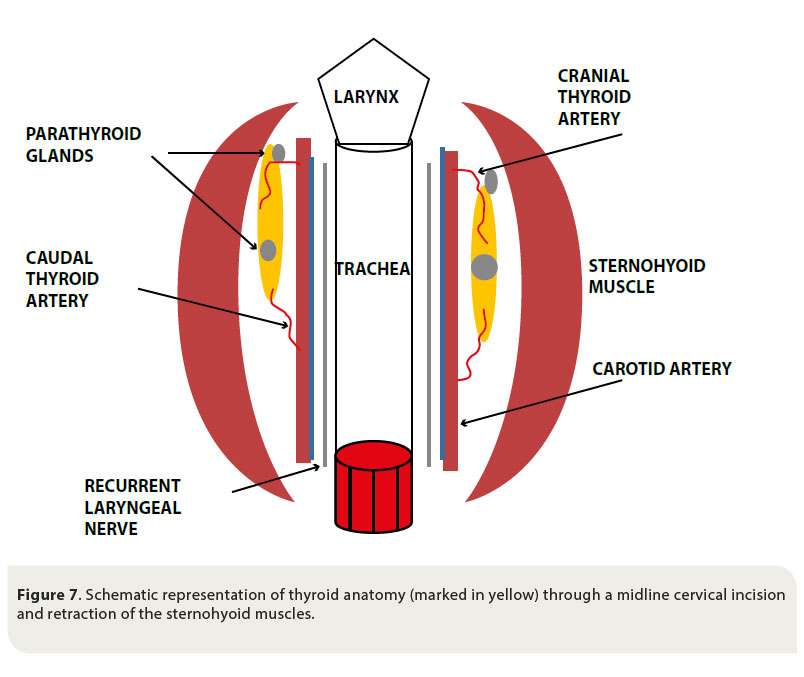
The parathyroid glands are closely attached to the thyroid gland and there are usually two for every thyroid lobe, one external on the cranial pole of the lobe, and one internal in the inner surface of the lobe, under the fibrous capsule or within the thyroid parenchyma. 44-45
The blood supply of the thyroid gland is provided by the cranial and caudal thyroid arteries. The cranial thyroid artery branches out from the carotid artery and the caudal thyroid artery arises from the brachiocephalic artery. Venous drainage is through the cranial thyroid vein, which drains into the jugular vein.44-45
Surgical preparation
In case of surgical treatment, the general condition of the dog must be evaluated as for every other surgical procedure through the appropriate haematology and biochemistry examinations. Moreover, due to extensive vascularisation of the thyroid and sometimes invasion of large vessels, extensive haemorrhage and sometimes severe blood loss are expected during thyroidectomy. This fact renders indispensable the hemostatic evaluation and the preparation for possible blood transfusion during or post surgery.32,43,46 Routine anaesthesia is induced considering that thyroid tumours are rarely functional.43 Furthermore, it is noted that even in cases of hyperthyroid dogs, achieving a euthyroid state prior to surgery is not necessary, in contrast to hyperthyroid cats. In any case of severe perioperative arrhythmias, however, or when severe hypertension is noted, the appropriate treatment should be instituted.31
Thyroidectomy 32,38,39,47
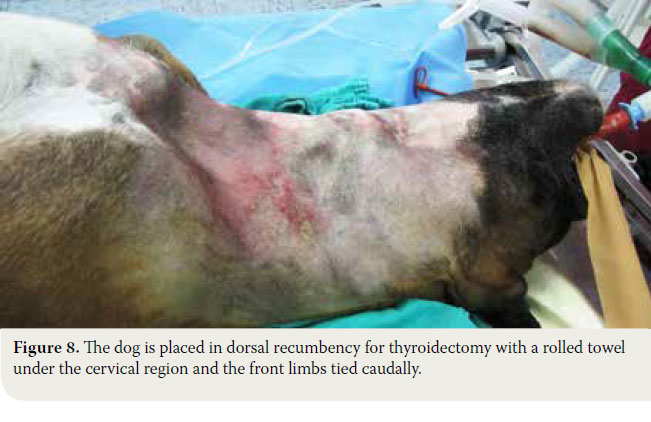
- Following induction of general anaesthesia, the dog is placed in dorsal recumbency, the front limbs are tied caudally, a folded towel is placed under the cervical region, in order to achieve mild hyperextension of the neck and a tube is placed in the oesophagus to facilitate orientation during surgery. The ventral aspect of the cervical region from caudal to the mandible to the manubrium is surgically prepared (Figure 8).
- A midline cervical incision is extended from the thyroid cartilage of the larynx to the level of the manubrium.
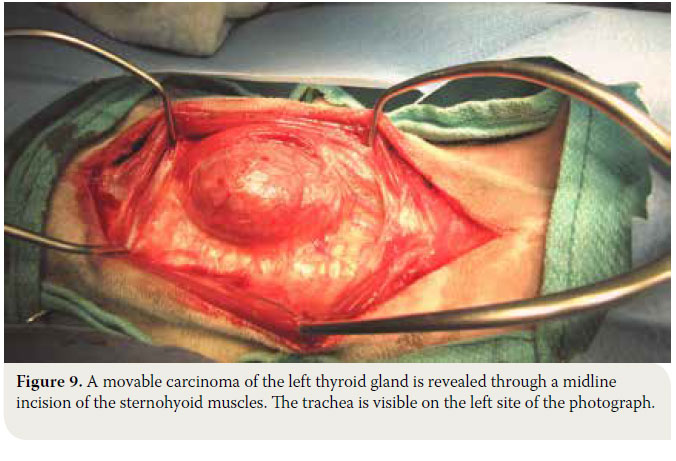
- The sphincter colli muscles are incised by scalpel to expose the paired sternohyoid muscles. Separation of the muscles is performed in the median raphe. Haemorrhage is controlled by diathermy or ligation of the small vessels that are located in the median raphe. The trachea is exposed and the thyroid glands are identified resting on the dorsal or dorsolateral aspects of the trachea near the thyroid cartilage (Figure 9).
- If the thyroid gland is not visible, the pair of sternothyroid muscles is retracted from the midline, the latter being located dorsally and on the inner aspect of the sternohyoid muscles. For an unimpeded view of the surgical site retraction of the sternohyoid and sternothyroid muscles is recommended with the placement of two Gelpi retractors in the cranial and caudal end of the incision.
- Both thyroid lobes are carefully observed, as well as their anatomical relationship to the surrounding structures.
- The recurrent laryngeal nerves are exposed, which lie along the trachea at the inner surface of the gland or in the fascia dorsal to the gland. During thyroidectomy, special attention is needed so as to avoid trumatising these nerves.
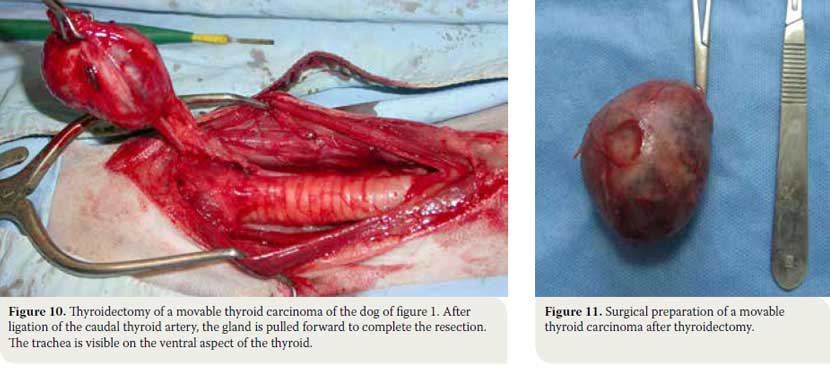
- The blood vessels surrounding the gland are ligated. The caudal thyroid artery is ligated and once the tissues dorsal to the gland are dissected, the gland is elevated and pulled forward so that the cranial thyroid artery can be identified and ligated. Ligations are done with synthetic absorbable suture no 3/0 or with vascular clips (Figures 10, 11). In cases when extensive haemorrhage is observed due to extensive neovascularisation, temporary unilateral ligation of the carotid artery and jugular vein may be necessary, and it is well tolerated by the animal.
- In cases of severely invasive carcinoma infiltrating the surrounding tissues and structures, such as the corresponding jugular vein, the carotid artery, the cervical sympathetic trunk and the recurrent laryngeal nerve, all of the above are excised and removed unilaterally en bloc, with no serious problems except for the development of unilateral Horner’s syndrome. In contrast, when carcinoma seems to be infiltrating the trachea and/or oesophagus or there is extensive neovascularisation, then the tumour is considered to be unresectable.
- When both glands are simultaneously affected, as long as they are movable, this can be addressed with bilateral thyroidectomy with or without preservation of the parathyroid glands. In cases of total parathyroidectomy, however, it is important to manage calcium homeostasis alterations.
- Midline closure of the muscles is done by 3/0 absorbable suture and routine subcutaneous tissue and skin closure is performed.
Postoperative monitoring and complications
Postoperative bleeding in dogs that underwent thyroidectomy can be severe. When bleeding is minor the application of cold packs on the area and bandaging the cervical region are recommended. In cases of severe bleeding blood transfusion is necessary and/or exploration of the surgical site to control the source of the bleeding.32,43,46
During thyroidectomy - if it has not previously been identified and properly protected - the recurrent laryngeal nerve can be traumatised. During the removal of large tumours, neurapraxia may occur due retraction of the nerve; dissection of the nerve may become necessary if it has been infiltrated by the neoplasm. In such cases and as long as the damage is unilateral, there are usually no respiratory complications. Moreover, voice disorders can be noted (change in tone, dysphonia, aphonia).46 Laryngeal paralysis is usually observed after bilateral injury to the recurrent laryngeal nerves during bilateral thyroidectomy. In such cases cricoarytenoid laryngoplasty is recommended.46 In cases of vagal nerve injury unilateral Horner’s syndrome can be observed, whereas during bilateral injury and/or dissection of the vagal nerve, megaoesophagus is to be expected.32,43
Following total bilateral thyroidectomy with concurrent removal of the parathyroid glands, most of the cases develop permanent hypoparathyroidism and consequent hypocalcemia.32 If there are clinical signs of hypocalcemia (restlessness, muscle tremors, seizures) calcium gluconate 10% is intravenously administered at a slow rate. In dogs with hypothyroidism and hypocalcemia, vitamin D and calcium supplements are administered for life with the first dose of vitamin D being offered twelve hours post-operatively.48
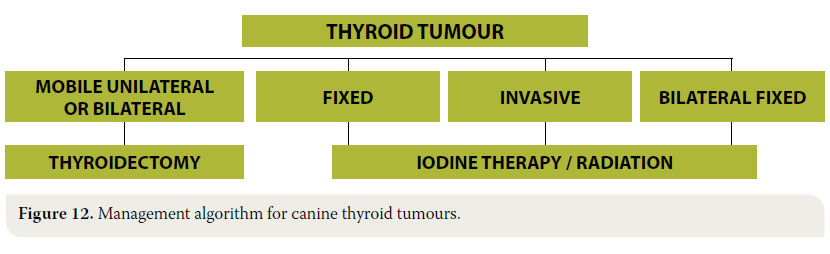
Moreover, after bilateral thyroidectomy there is a possibility for hypothyroidism, and for that reason, thyroid hormone concentrations must be frequently measured in blood serum. In such cases hormone replacement medications are provided such as sodium L-thyroxine or levothyroxine.46 The management algorithm for thyroid tumours is summarised in Figure 12.
Prognosis
In cases of thyroid adenoma the prognosis is excellent, considering that total surgical extraction of the tumour results in clinical cure.
Prognosis of thyroid carcinoma is associated with the size and invasiveness of the tumour, as well as the presence of metastatic lesions, whereas no correlation has been shown between vascular density and survival time.40 In two studies of dogs with movable, noninvasive carcinomas that underwent surgical excision a median survival time of one to three years and seven months were reported respectively. 3,40 Moreover, another big study in 82 dogs with thyroid carcinoma revealed that when the twenty dogs with movable carcinoma without indications of metastasis underwent surgical excision of the tumours, median survival time was 36 months.7 In dogs with inoperable carcinoma, in which no other treatment was undertaken, a clinical study showed that out of eight dogs, five survived for about six months and two for at least one year, whereas in another study median survival time was three months.9,10 Recently in a study of fifteen dogs with movable bilateral thyroid carcinoma that underwent bilateral thyroidectomy with or without concurrent excision of the parathyroid glands it was noted that median survival time was 38.3 months; in this study eleven dogs presented with hypocalcemia during the immediate postoperative period, and at the end of the study seven received treatment for hypocalcemia, whereas in eight dogs medical treatment was administered for hypothyroidism. 49 Finally, in cases of medullary carcinoma, it is not known if prognosis is more favorable compared to thyroid adenocarcinoma. It is worthy of note that medullary carcinoma is better encapsulated and is less invasive for the surrounding tissues compared to adenocarcinoma.10
Radiation therapy
Radiation therapy can be sufficiently effective in cases of extensively invasive and inoperable tumours and it is used either as monotherapy or in combination with surgical treatment, which is undertaken later, after the tumour has regressed. There are several radiotherapy protocols for fractionated or hypofractionated radiation therapy.8,50 In a large study of 25 dogs with extensively invasive thyroid carcinoma with no metastases that underwent hypofractionated radiation therapy, survival percentages regardless of the tumour progression reached 80% one year post radiotherapy and 72% three years later. The time during which the tumours reached their smallest size varied between 8-22 months, whereas only 28% of the dogs had metastatic lesions in other sites.8 In a different smaller study performed in dogs with invasive thyroid carcinoma, which underwent fractionated radiotherapy, median survival time was two years.49 Both hypofractionated as well as fractionated protocols offer excellent results regarding survival time and the prevention of metastatic lesions. 8,50
Radioactive iodine treatment (131I)
Treatment with131I is recommended in cases of invasive carcinoma or in the presence of metastatic sites and it can be combined with surgical management or used as monotherapy. Published studies showed that it can significantly prolong survival time.9,13 In a large study, in 65 dogs with thyroid carcinoma, 43 dogs received131I (1-3 sessions of131I in a dose of 555-1850 MBq), in combination with surgery or not, whereas the rest of the dogs received no treatment. Median survival times were 34, 30, and 3 months, respectively.9
Chemotherapy
Chemotherapy is used in dogs with invasive carcinoma with or without metastasis combined with or without surgery. Its effectiveness, however, is doubted for the time being. In a study in which doxorubicin was administered as monotherapy, in 40% of dogs carcinoma had regressed to less than half its original size and median survival time reached 37 weeks.51 In another study in which cisplatin was used, 54% of dogs had regression in tumour size to less than half the original size and median survival time was 322 days. However, in most of these dogs doxorubicin had been previously administered, and this makes any estimation of the effect of cisplatin challenging. Τhe remaining 46% of the dogs had a median survival time of 98 days.14 In a recent study in 44 animals chemotherapy was instituted with several chemotherapeutic medications (carboplatin, cisplatin, doxorubicin or gemcitabine) as monotherapy or combined with surgical treatment. Median survival times of animals between these two groups did not differ significantly.52
> References
1. Brodey RS, Kelly DF. Thyroid neoplasms in the dog. A clinicopathologic study of fifty-seven cases. Cancer 1968, 22: 406-416.
2. Birchard SJ, Roesel OF. Neoplasia of the thyroid gland in the dog: a retrospective study of 16 cases. J Am Anim Hosp Assoc 1981, 17: 369-372.
3. Hararι J, Patterson JS, Rosenthal RC. Clinical and pathologic features of thyroid tumors in 26 dogs. J Am Vet Med Assoc 1986, 188: 1160-1164.
4. Wucherer KL, Wilke V. Thyroid Cancer in Dogs: An Update Based on 638 Cases (1995-2005). J Am Anim Hosp Assoc 2010, 46: 249-254.
5. Loar AS. Canine thyroid tumors. In Current Veterinary Therapy IX. Kirk RW (ed). WB Saunders: Philadelphia, 1986, pp. 1033-1039.
6. Barber LG. Thyroid tumors in dogs and cats. Vet Clin North Am Small Anim Pract 2007, 37: 755-773.
7. Klein MK, Powers BE, Withrow SJ, Curtis CR, Straw RC, Ogilvie GK, Dickinson KL, Cooper MF, Baier M. Treatment of thyroid carcinoma in dogs by surgical resection alone: 20 cases (1981-1989). J Am Vet Med Assoc 1995, 206: 1007-1009.
8. Théon AP, Marks SL, Feldman ES, Griffey S. Prognostic factors and patterns of treatment failure in dogs with unresectable differentiated thyroid carcinomas treated with megavoltage irradiation. J Am Vet Med Assoc 2000, 216: 1775-1779.
9. Worth AJ, Zuber RM, Hocking M. Radioiodide (131) I therapy for the treatment of canine thyroid carcinoma. Aust Vet J 2005, 83: 208-14.
10. Carver JR, Kapatkin A, Patnaik AK. A Comparison of medullary thyroid carcinoma and thyroid adenocarcinoma in dogs: A retrospective study of 38 cases. Vet Surg 1995, 24: 315-319.
11. Leav I, Schiller AL, Rijnberk A, Legg MA, Der Kinderen PJ. Adenomas and Carcinomas of the Canine and Feline Thyroid. Am J Pathol 1976, 83: 61-122.
12. Hayes HM, Fraumeni JF. Canine thyroid neoplasms: epidemiologic features. J Natl Cancer Inst 1975, 55: 931-934.
13. Turrel JM, McEntee MC, Bruke BP, Page RL. Sodium iodide I 131 treatment of dogs with nonresectable thyroid tumors: 39 cases (1990-2003). J Am Vet Med Assoc 2006, 229: 542-548.
14. Fineman LS, Hamilton TA, Gortari A, Bonney P. Cisplatin chemotherapy for treatment of thyroid carcinoma in dogs: 13 cases. J Am Anim Hosp Assoc 1998, 34: 109-112.
15. Benjamin SA, Saunders WJ, Angleton GM, Lee AC. Radiation carcinogenesis in dogs irradiated during prenatal and postnatal development. J Radiat Res 1991, 32: 86-103.
16. Verschueren CP, Rutteman GR, Vos JH, Van Dijk JE, De Bruin TW. Thyrotrophin receptors in normal and neoplastic (primary and metastatic) canine thyroid tissue. J Endocrinol 1992, 132: 461-468.
17. Benjamin SA, Stephens LC, Hamilton BF, Saunders WJ, Lee AC, Angleton GM, Mallinckrodt CH. Associations between lymphocytic thyroiditis, hypothyroidism, and thyroid neoplasia in beagles. Vet Pathol 1996, 33: 486-494.
18. Suarez HG, du Villard JA, Severino M, Caillou B, Schlumberger M, Tubiana M et al. Presence of mutations in all three ras genes in human thyroid tumors. Oncogene 1990, 5: 565-570.
19. Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer 2006, 6: 292-306.
20. Verschueren CP. Flow-Cytometric DNA Ploidy analysis in primary and metastatic canine thyroid carcinomas. Anticancer Res 1991, 11: 1755-1761.
21. Bertazzolo W, Giudice C, Dell’Orco M, Caniatti M. Paratracheal cervical mass in a dog. Vet Clin Pathol 2003, 32: 209-212.
22. Almes KM, Heaney AM, Andrews JA. Intracardiac ectopic thyroid carcinosarcoma in a dog. Vet Pathol 2008, 45: 500-504.
23. Lantz GC, Salisbury SK. Surgical excision of ectopic thyroid carcinoma involving the base of the tongue in dogs: three cases (1980-1987). J Am Vet Med Assoc 1989, 195: 1606-1608.
24. Broome MR, Peterson ME, Walker JR. Clinical features and treatment outcomes of 41 dogs with sublingual ectopic thyroid neoplasia. J Vet Intern Med 2014, 28: 1560-1568.
25. Liptak JM, Kamstock DA, Dernell WS, Ehrhart EJ, Rizzo SA, Withrow SJ. Cranial mediastinal carcinomas in nine dogs. Vet Comp Oncol 2008, 6: 19-30.
26. Constantino-Casas F, Rodriguez-Martinez HA, Gutierrez Diaz- Ceballos ME. A case report and review: the gross, histological and immunohistochemical characteristics of a carcinoma of ectopic thyroid in a dog. Br Vet J 1996, 152: 669-672.
27. Kiupel M, Capen C, Miller M, Smedley R. Histological classification of the endocrine system of domestic animals Washington. Armed Forces Institute of Pathology, 2008, pp. 64-68.
28. Patnaik AK, Lieberman PH. Gross, histologic, cytochemical and immunocytochemical study of medullary carcinoma in sixteen dogs. Vet Pathol 1991, 28: 223-233.
29. Susaneck SJ. Thyroid tumors in the dog. Compend Cont Educ Pract Vet 1983, 5: 35-38.
30. Taeymans O, Peremans K, Saunders JH. Thyroid imaging in the dog: current status and future directions. J Vet Intern Med 2007, 21: 673-684.
31. Simpson AC, McCown JL. Systemic hypertension in a dog with a functional thyroid gland adenocarcinoma. J Am Vet Med Assoc 2009, 235:1474-1479.
32. Seguin B, Brownlee L, Walsh PJ. Endocrine system. In Veterinary Surgical Oncology. Kudnig ST, Sequin B(eds). Wiley-Blackwell: Chichester, 2012, pp. 405- 441.
33. Wisner ER, Nyland TG. Ultrasonography of the thyroid and parathyroid glands. Vet Clin North Am Small Anim Pract 1998, 28: 973-991.
34. Weber AL, Randolph G, Aksoy FG. The thyroid and parathyroid glands. CT and MR imaging and correlation with pathology and clinical findings. Radiol Clin North Am 2000, 38: 1105-1129.
35. Feldman N. Canine thyroid tumors and hyperthyroidism. In Canine and Feline Endocrinology and Reproduction. Feldman N (ed). 3rd ed. Saunders: Philadelphia, 2003, pp. 219-249.
36. Taeymans O, Penninck DG, Peters RM. Comparison between clinical, ultrasound, CT, MRI, and pathology findings in dogs presented for suspected thyroid carcinoma. Vet Radiol Ultrasound 2013, 54: 61-70.
37. Taeymans O, Schwarz T, Duchateau L, Barberet V, Gielen I, Haskins M, van Bree H, Saunders J. Computed tomographic features of the normal canine thyroid gland. Vet Radiol Ultrasound 2008, 49: 13-19.
38. Liptak JM. Canine thyroid carcinoma. Clin Tech Small Anim Pract 2007, 22: 75-78.
39. Flanders JA. Surgical therapy of the thyroid. Vet Clin North Am Small Anim Pract 1994 , 24: 607-621.
40. Kent MS, Griffey SM, Verstraete FJ, Naydan D, Madewell BR. Computerassisted image analysis of neovascularization in thyroid neoplasms from dogs. Am J Vet Res 2002, 63: 363-369.
41. Sullivan M, Cox F, Pead MJ, McNell P. Thyroid tumours in the dog. J Small Anim Pract 1987, 28: 505-512.
42. Owen LN. TNM Classification of Tumours in Domestic Animals. 1st ed. World Health Organization: Geneva, 1980, pp. 51-53.
43. Lunn KF, Page RL. Tumors of the endocrine system. In Withrow and Mac- Ewen’s Small Animal Clinical Oncology. Withrow SJ, Vail DM, Page RL (eds) 5th ed. Elsevier: St Louis, 2013, pp. 504-531.
44. Michail S. Comparative Anatomy of the Domestic Animals. 2nd edn. D Kyriakidis: Thessaloniki, 2015.
45. Hullinger RL. The endocrine system. In Miller’s Anatomy of the Dog. Evans HE (ed). 3rd ed. WB Saunders Co: Philadelphia, 1993, pp. 559-585.
46. Radlinsky MG. Thyroid surgery in dogs and cats. Vet Clin Small Anim 2007, 37: 789-798.
47. Fukui S, Endo Y, Hirayama K, Taniyama H, Kadosawa T. Identification and preservation of the parathyroid gland during total thyroidectomy in dogs with bilateral thyroid carcinma: a report of six cases. J Vet Med Sci 2015, 77: 747-751.
48. Kornegay JN. Hypocalcemia in dogs. Compend Cont Educ Pract Vet 1982, 4: 103-110.
49. Tuohy JL, Worley DR Withrow SJ. Outcome following simultaneous bilateral thyroid lobectomy for treatment of thyroid gland carcinoma in dogs: 15 cases (1994–2010). J Am Vet Med Assoc 2012, 241: 95-103.
50. Pack L, Roberts R, Dawson SD, Dookwah HD. Definitive radiation therapy for infiltrative thyroid carcinoma in dogs. Vet Radiol Ultrasound 2001, 42: 471-474.
51. Jeglum KA, Whereat A. Chemotherapy of canine thyroid carcinoma. Compend Cont Educ Pract Vet 1983, 5: 96-98.
52. Nadeau ME, Kitchell BE. Evaluation of the use of chemotherapy and other prognostic variables for surgically excised canine thyroid carcinoma with and without metastasis. Can Vet J 2011, 52: 994-998.










 PDF Download
PDF Download The present editorial was drawn up with regard to the publication of guidelines for veterinary dentistry, issued by the World Small Animal Veterinary Association (WSAVA). It is a document of major importance, covering 165 pages. The Hellenic Companion Animal Veterinary Society took the initiative to translate the guidelines into the Greek language, with a view to distributing the document to its members in the 10th anniversary Forum in March 2019.
The present editorial was drawn up with regard to the publication of guidelines for veterinary dentistry, issued by the World Small Animal Veterinary Association (WSAVA). It is a document of major importance, covering 165 pages. The Hellenic Companion Animal Veterinary Society took the initiative to translate the guidelines into the Greek language, with a view to distributing the document to its members in the 10th anniversary Forum in March 2019.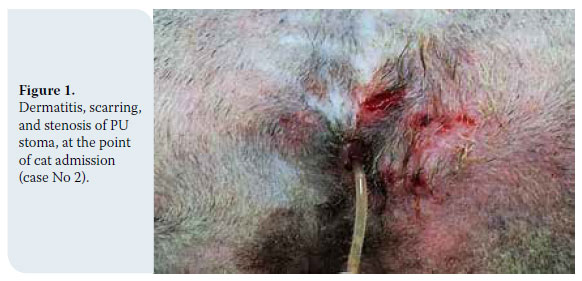
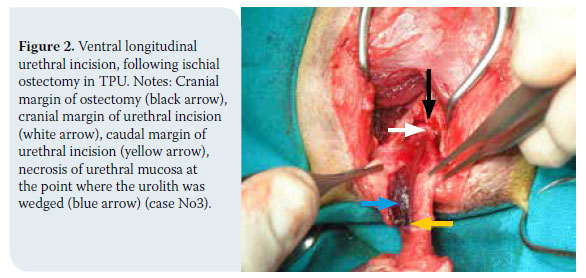
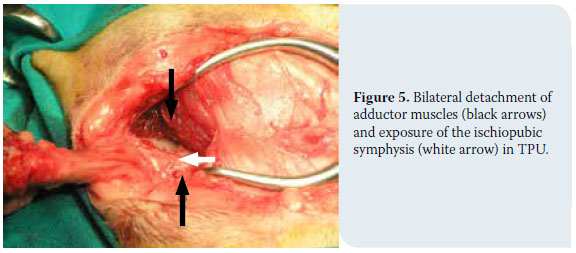
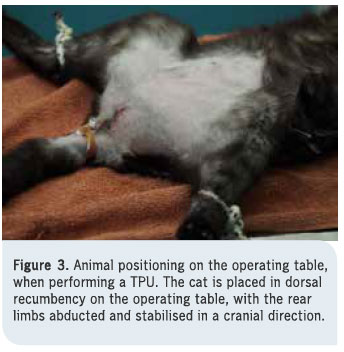 1. Cat positioning and antisepsis
1. Cat positioning and antisepsis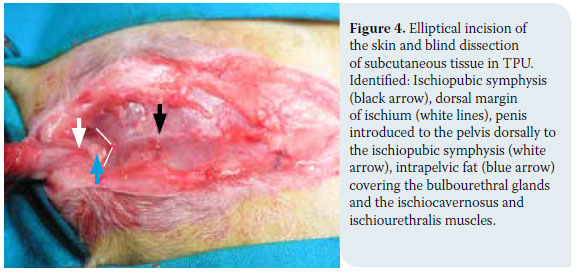
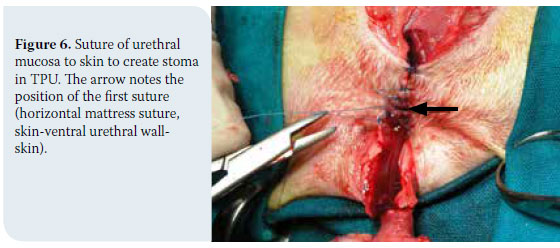
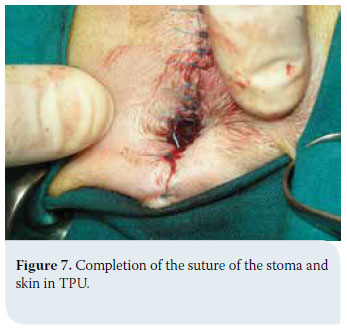
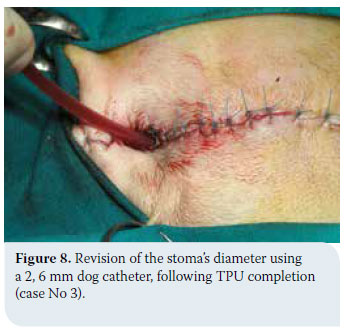




 In cases of thyroid adenoma, surgical excision of neoplastic tissue is the treatment of choice and leads to clinical cure.6,32 Regarding thyroid carcinoma, treatment options depend on factors including tumour size, the extent of surrounding tissue infiltration, the presence of metastatic lesions and the possibility of using alternative treatment modalities (radiation therapy, chemotherapy).43 In general, surgical treatment is indicated in cases of small (<7cm) and movable neoplasms or tumours with superficial infiltration of surrounding tissues, but it is contraindicated in cases of severely invasive and fixed tumours (Figure 6).6,7,10,32 In many cases, surgical exploration of the carcinoma is necessary in order to identify the extent of surrounding tissue invasion. In cases where metastatic lesions are present at the time of diagnosis, treatment is palliative and includes monotherapy or a combination of surgical excision and radiation therapy. In cases when diagnostic imaging has not demonstrated the presence of metastasis, selection of treatment is based on whether the tumour is movable or fixed. If it is movable, surgical excision by unilateral or more rarely bilateral thyroidectomy is undertaken, combined or not with radiotherapy. When the tumour is fixed, surgical treatment is contraindicated and radiotherapy and radioactive iodine administration are recommended.32
In cases of thyroid adenoma, surgical excision of neoplastic tissue is the treatment of choice and leads to clinical cure.6,32 Regarding thyroid carcinoma, treatment options depend on factors including tumour size, the extent of surrounding tissue infiltration, the presence of metastatic lesions and the possibility of using alternative treatment modalities (radiation therapy, chemotherapy).43 In general, surgical treatment is indicated in cases of small (<7cm) and movable neoplasms or tumours with superficial infiltration of surrounding tissues, but it is contraindicated in cases of severely invasive and fixed tumours (Figure 6).6,7,10,32 In many cases, surgical exploration of the carcinoma is necessary in order to identify the extent of surrounding tissue invasion. In cases where metastatic lesions are present at the time of diagnosis, treatment is palliative and includes monotherapy or a combination of surgical excision and radiation therapy. In cases when diagnostic imaging has not demonstrated the presence of metastasis, selection of treatment is based on whether the tumour is movable or fixed. If it is movable, surgical excision by unilateral or more rarely bilateral thyroidectomy is undertaken, combined or not with radiotherapy. When the tumour is fixed, surgical treatment is contraindicated and radiotherapy and radioactive iodine administration are recommended.32




 > Case report
> Case report