> Abstract
The owners of a 3-month-old female DSH cat witnessed her eating raw mushrooms of the species Boletus edulis, Boletus aereus and Amanita caesarea. These mushrooms are edible for humans and highly prized in various cuisines. Vomiting, hypersalivation, horizontal head oscillation and limb muscle tremor were developed within 6 hours. Two days later the cat was admitted due to depression and anorexia, while the neurologic signs had subsided. Dehydration, depression, lymphopenia, increased serum urea nitrogen concentration, proteinuria and bilirubinuria were detected. During the 5-day-hospitalisation period, treatment comprised of intravenous fluids, and per os vitamin E and hepatoprotectants (SAMe – vitamin Ε – vitamin C – silibinin complex). Due to mucohaemorrhagic diarrhoea present on the first day of hospitalisation, ampicillin and sucralfate were subsequently added. The kitten recovered completely a week later and was still healthy 8 months later. Mushrooms in general, are classified as edible or poisonous; the latter could be hepatotoxic, neurotoxic, nephrotoxic, gastroenterotoxic, muscarinic or coprinoid. This basic classification based on human experience may not apply to other species, and consequently “edible” mushroom species may be potentially toxic for animals. In addition, in many cases of mushroom ingestion in animals, the species involved remained unidentified. Thus, this case report describes presumed poisoning from three identified mushrooms, Boletus edulis, Boletus aereus and/or Amanita caesarea, which are considered edible for humans, but caused gastrointestinal, hepatic and neurologic signs in a cat. Prognosis in these cases may be favourable, if early supportive care is instituted.
> Introduction
Mushrooms are classified as edible or poisonous; the former can be edible by all species or only by humans but toxic for animals, whilst the latter can be hepatotoxic, neurotoxic, nephrotoxic, gastroenterotoxic, muscarinic or coprinoid.1-3 There is also another classification that may be useful for the clinician, based on the time of onset of clinical signs from exposure; mushrooms with a toxic latent period up to 3 hours from ingestion (self-limiting, not life-threatening), up to 6 hours from ingestion (lifethreatening) and up to 24 hours from ingestion4. The toxicity of a mushroom depends on its toxin and/or the dose consumed. Hepatotoxic mushrooms (e.g. Amanita ocreata, A. phalloides) contain mainly cyclopeptides (amatoxins) causing acute liver failure and death in humans and animals. A. phalloides (“death cap”) is considered to be the most toxic mushroom worldwide.2 Neurotoxic mushrooms contain hydrazines (e.g. Gyromitra spp.), isoxazoles (e.g. A. pantherine, A.muscaria), psilocin and psilocybin (e.g. Psilocybe spp., Panaeolus spp., Conocybe spp., Gymnopilus spp.). Nephrotoxic mushrooms (e.g. Cortinarius sp.) contain a bipyridyl toxin (orellanine), which is thought to be their main toxin. Most of the human and animal cases with Cortinarius sp. intoxication eventually develop renal failure.2 Gastroenterotoxic mushrooms (e.g. Agaricus sp., Boletus sp.) contain a variety of toxins, the majority of which have not been identified, but they mainly cause gastrointestinal (GI) signs (GI irritants). Muscarinic mushrooms (e.g. Inocybe spp., Clitocybe spp.) contain muscarine; the animals present clinical signs characterised by the acronym SLUDDE (Salivation, Lacrimation, Urination, Diarrhea, Dyspnea and Emesis).2 The coprinoid mushrooms are the coprine-containing Coprinopsis spp. Toxicosis in humans occurs with the simultaneous consumption of these mushrooms and alcohol (alcohol-induced toxicosis); thus, these poisonings occur exclusively in humans.1 Ultimately, all toxic mushrooms, regardless of their classification, may lead to multi-systemic manifestations.
This report describes a case of mushroom toxicosis in a cat where the species involved were identified as Boletus edulis, B. aereus and Amanita caesarea, which are highly regarded edible species in human cuisine. Mushroom toxicoses have not been described thoroughly in animals and especially in cats; consequently, the current case report could promote knowledge on feline mushroom intoxication, on potential feline toxic mushroom species and on mushroom toxicosis management.
 > Case report
> Case report
A 3-month-old female DSH non-vaccinated cat was observed eating pieces of three different mushroom species collected by the owner for consumption (Figure 1). The mushrooms were identified as Boletus edulis, Boletus aereus, and Amanita caesarea by the owner, a chemist and experienced mushroom collector and by A. Dinopoulos DVM, PhD. A. Dinopoulos is a professor in anatomy and histology (therefore a morphologist). He has a long experience in hunting mushrooms and he is the writer of a relevant book; thus he can be considered as an expert in mushroom identification, taking into account the absence of this specialty in Greece. Within 6 hours, hypersalivation, vomiting, horizontal head oscillation and limbs muscle tremor were the initial presenting signs. On the following day, the cat developed anorexia and depression, and she did not visit her litterbox. Forty-eight hours after ingestion the cat was admitted to our university clinic due to persistent anorexia and depression. No neurologic signs were present at that time. Careful questioning of the owner to possible exposure of the kitten to other toxicants (e.g. food, plants, insecticides, pesticides, medications, detergents) did not reveal any exposure.
On physical examination, depression, dehydration and bilateral third eyelid protrusion were detected, while neurologic examination did not reveal any abnormalities concerning mental status, posture, gait, nociception, postural reactions and cranial nerve assessments.
In standard haematology and clinical chemistry testing, lymphopenia, thrombocytopenia, and elevation of serum urea nitrogen (BUN) concentration were detected (Table 1). Urinalysis revealed bilirubinuria and proteinuria. Urine specific gravity was 1.060, whilst urine sediment microscopic examination and urine protein/creatinine ratio were normal.
Serology for FeLV/FIV infection through ELISA snap test (Snap® Combo FeLV/FIV, IDEXX Laboratories Inc., Maine, USA) and an agglutination test were negative. Moreover, anal mucosal swab cytology revealed neutrophilic inflammation. Neither parasitic elements nor fungal spores were found on fecal examination (sedimentation and flotation method). Also, buffy-coat cytology was normal.
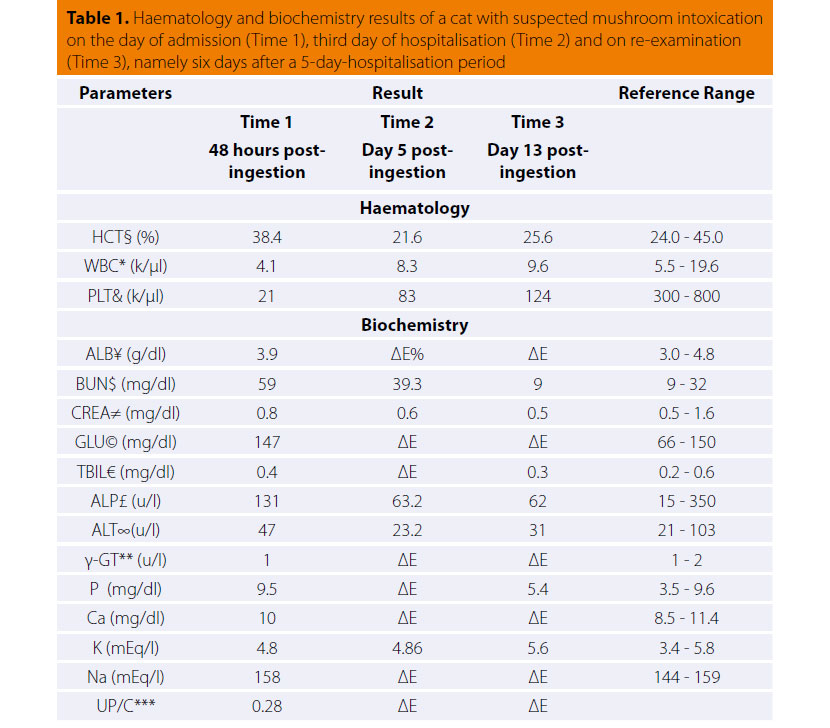
§Haematocrit; *White blood cells; &Platelets; ¥Albumin; $Blood urea nitrogen, ≠Creatinine;
©Glucose; €Total bilirubin; £Alkaline phosphatase; ∞Alanine transfera; **γ Glutamyl transferase;
***Urine protein/creatinine ratio; %Not done
Intravenous fluids [Half-Strength Saline (1:1 NaCl 0.9%, Dextrose 5%)], per os vitamin E (Eviol®, G.A. Pharmaceuticals Ltd., Athens, Attica, Greece) in the dose of 8mg/kg q24h and per os hepatoprotectants, a complex of S-adenosyl-methionine, vitamin Ε, vitamin C and silibinin (Samylin®, VetPlus, Lytham, UK) in the dose of 20mg/kg q24h, were administered for the dehydration as well as any potential hepatotoxicity, suspected due to billirubinuria, respectively. The cat developed mucohaemorrhagic diarrhoea on the first day of hospitalisation and intravenous ampicillin (Begalin®, Pfizer Hellas Ltd., Neo Psychiko, Attica, Greece) in the dose of 20mg/ kg q8h and per os sucralfate (Peptonorm®, Uni- Pharma S.A. Pharmaceutical Laboratories, Kifissia, Attica, Greece) in the dose of 1g/30kg q8h, were added.
The cat was discharged from the clinic five days after admission in good general condition and appetite, but still showing a mild diarrhoea. Vitamin E, hepatoprotectants, ampicillin and sucralfate were prescribed per os for 6 more days. On reexamination, 6 days after discharge, she was fully recovered. Physical, neurologic and laboratory examination (haematology, serum biochemistry, urinalysis, parasitological fecal examination) were normal and remained normal on re-examination eight months later.
> Discussion
Mushroom intoxications in animals, and especially in cats, are underreported.2 Only six feline incidents per year during a four-year period have been recorded by the American Society for the Prevention of Cruelty to Animals (ASPCA) – Animal Poison Control Center (APCC) in the USA, while canine cases in the same period number 400. Ιn the majority of feline mushroom toxicoses reported, mushrooms were characterised as of “unknown origin” and were not identified.2 In addition, the North American Mycological Association (NAMYCO) has recorded 21 feline mushroom intoxications over a forty-year period (1974 – 2016) in the USA,5-8 whilst 28 enquiries have been recorded by the Veterinary Poisons Information Service (VPIS) through an eighteenyear period (1999 – 2016) in the UK9,10 plus two short reports in the veterinary toxicology literature.1,11 Cats are potentially susceptible to toxicosis from all edible and non-edible mushrooms;2 however, there is no information to specify the toxic mushroom species for cats or their toxic doses. Indeed, there are only two detailed reports concerning three cats with mushroom intoxication, but the mushroom species in these cases were unfortunately not identified.12,13 Amanita spp. and especially A. ocreata, as well as Conocybe sp., Galerina sp. and some unknown species have been reported to cause hepatotoxicity in cats.5,8,13 Amanita pantherina and Amanita muscaria, containing ibotenic acid and muscimol, respectively, as well as Psilocybin spp. and Inocybe spp. have been recorded to be neurotoxic for cats.5,7,8,9,11 Muscimol causes intoxication in humans and cats called “pantherine-muscaria” syndrome, which is characterised by mydriasis, dryness of the mouth, ataxia, disorientation, euphoria, dizziness and tiredness occurring within 30 minutes to 2 hours after consumption, and followed by full recovery within 1 to 2 days.11 Death, however, has been reported following Amanita muscaria consumption in cats.7,9 Cortinarius orellanus may cause renal tubular epithelium damage in cats.11 Agaricus spp.1,5 and Russula spp.1 have been incriminated as gastroenterotoxic mushrooms. Russula spp. have a shellfish odor, which may make them attractive to cats.1 Moreover, consumption of Tricholomapardinum and/or Paxillusatrotomentosus 5 and Armillaria spp. (especially Armillaria gallica) have been reported to cause GI distress in animals10 possibly due to the sesquiterpene aryl ester compound of the latter. Muscarinic mushroom toxicosis was suspected in two cats with acute dyspnoea, open-mouth breathing, cyanosis and hypersalivation followed by vomiting, diarrhea, miosis, bradycardia, tachypnoea, azotaemia, and finally, full recovery.12 At last, intoxication in cats has been identified by Coprinopsis atramentaria var. crassivelata and Pluteus cinereofuscus targeting various body systems.6,8,10
Amanita caesarea has not been reported as a potentially toxic mushroom, but, given the signs caused by other Amanita species, and as was seen also in the cat of this report, it may be hypothesised that the neurologic signs could have been caused by Amanita caesarea consumption. Furthermore, amatoxins (i.e. α-amanitin), possibly contained in Amanita caesarea of our report, targets hepatocytes, crypt cells, and proximal convoluted tubules of the kidney via inhibition of protein synthesis.14 Additionally, Boletus spp. are considered to have gastroenterotoxic properties and may cause intoxication in cats. All Boletus spp., including Boletus edulis and Boletus aereus, are considered edible and highly prized by humans, however, Boletus spp. have been classified as GI irritants,2,15 because they contain substances that cause GI upset. The mechanism of action is hypothesised to be idiosyncratic or allergic.15 In addition, it is suspected that Boletus spp. contain significant amounts of muscarine.16,17 Muscarine binds to cholinergic receptors resulting in effects on smooth muscles, exocrine glands and the cardiovascular system; thus, muscarine can cause GI distress manifesting as increased gastric tract peristalsis and diarrhea.3 Consequently, gastrointestinal signs in this case may be attributed to Boletus edulis and/or Boletus aereus consumption. In the present case, the owners consumed the mushrooms overall, without presenting any clinical sign of toxicity. Rare cases of allergic reactions to B. edulis have been described in humans,18 as well as trehalose dysanexia, in which this sugar is not absorbed due to deficiency of trehalase.19 Moreover, sixteen cases of human intoxication by B. edulis have been recorded, where GI distress occurred 6-7 hours after mushroom consumption.5,7,20 Another syndrome associated with B. edulis is alcoholinduced GI distress in susceptible individuals in up to 5 hours after consumption, but dissimilar to the Coprinoid Alcohol-Induced Syndrome (also called Antabuse syndrome).21
Occasionally, spoiled mushrooms (contaminated by bacteria) produce illness3 rather than toxins present in the mushrooms. In this case, however, the mushrooms were washed and well preserved prior to intended consumption by the cat’s owners.
In general, mushrooms can cause a variety of non-specific clinical and clinicopathologic signs, which make diagnosis of a mushroom-specific toxicosis difficult. Lymphopenia, in this case, was attributed to a stress leukogram, whilst urea nitrogen elevation could have been a consequence of dehydration. Thrombocytopenia was probably the result of sampling difficulties in this kitten, an assumption supported by the evidence of platelet aggregates found on blood smear examination. Also, proteinuria could have been a false positive finding in the chromatographic dipstick since the UPC ratio was normal. Finally, although bilirubinuria has not been comprehensively studied in feline medicine, in this case it could have been the result of hepatotoxicosis or a reactive hepatic consequence of the gastrointestinal inflammation. Nevertheless liver enzyme activities were within normal limits perhaps due to their short half-life; serum half-life of alanine is aminotrasferase < 24 hours (about 3-4 hours) and of alkaline phosphatase 6h in cats.
Differential diagnosis in this case of GI distress accompanied by possible hepatic failure and neurologic signs in a young cat, include porto-systemic shunt and associated hepatic encephalopathy, bacterial gastrointestinal infection, as well as intoxication by food (including toxins such as aflatoxin, gyromitrin), plants (lily toxicosis, cocklebur, cycad palm, ricin, abrin, marijuana), pesticides (carbamates, organophosphates), microcystins in cyanobacteria, copper, zinc and acetaminophen overdose,3 and amphetamines. The cat of this study presented neurologic as well as GI and systemic signs. Of great importance was thought to be the evidence of mushrooms consumption observed by the owner, and the lack of possible exposure of the cat to other toxic substances. Furthermore, infectious diseases were ruled out grossly through laboratory investigation and the information about her lifestyle, being exclusively an indoor cat. Ultimately, response to treatment and favourable outcome excluded any congenital anomalies. Therefore, mushroom toxicosis was considered to be the most likely diagnosis. Mushroom species identification is of great importance as well and although the species involved were reliably identified, it is difficult to determine whether one or more of the species involved resulted in the presenting signs.
In mushroom poisoning, identification is undertaken by morphological (mainly sporological) and/or biochemical (toxicological) analysis. Etiologic diagnosis can be established by identification of toxins in serum or urine samples with an ELISA based method22 or highperformance liquid chromatography,23 however, these methods are not routinely performed in veterinary medicine. Amanitines are detectable in the urine of dogs for hours and in humans for up to three days after mushroom consumption; this may indicate ongoing intestinal absorption, intestinal reabsorption or reduced renal amanitin elimination due to toxic kidney damage.24 In plasma, the half-life of amanitines is 25-50 minutes, while it cannot be detected 24 hours after exposure.14 Consequently, any vomitus should be examined for the presence of mushrooms,2 while mushroom samples should be placed in paper (not plastic bags) or ideally wrapped in wax paper.1 Muscarine can be detected in urine and GI content, but analysis is not routinely offered by veterinary diagnostic laboratories. However, a positive response to a therapeutic test with atropine is of great diagnostic importance.3 In this case, an etiologic diagnosis based on the detection of mushroom toxins by specific techniques could not be established due to the delayed admission of the kitten to our clinic and the lack of a mushroomspecific toxicological laboratory in Greece. Also, due to the delayed presentation, sporological examination based on microscopic examination of the clinical material was not performed in our case.
For the majority of mushroom toxins, there are no antidotes with the exception of muscarine, for which atropine reverses the cholinergic effects. Treatment is supportive with management of hypovolemic shock, dehydration, hepatotoxicity, neurotoxicity or other clinical signs. In general, activated charcoal may not increase decontamination due to the rapid onset of clinical signs.3 Silibinin was administered in our case because of suspected hepatotoxicity; it is the main component of silymarin, extracted from the common milk of thistle, Silybum marianum, and it reduces the uptake of amanitines into hepatocytes. A complex of silibinin with phosphatidyl choline (lecithin), known as silipide, has been suggested for amatoxin poisoning. It has four to ten times better oral bioavailability than pure silibinin, but has not been tested in clinical cases in animals.25
Prognosis depends on a variety of factors such as the age of the patient, the quantity of mushroom ingested, the mushroom species and time of treatment initiation, as well as the specific measures undertaken.3 In this report, despite the young age of this cat, which was a poor prognostic factor, the cat survived and responded favourably to the supportive treatment, perhaps reflecting the small amount of mushroom consumed and the appropriate management protocol instituted. It is important to note that gastroenterotoxic mushroom intoxication is rarely fatal.3
In conclusion, this is the first report of ingestion of the edible mushroom species Boletus edulis, Boletus aereus and Amanita caesarea causing toxicosis in cats. Until more information is available, any mushroom species should be considered potentially toxic for cats. The need for a global or national mushroom toxicosis case registry database for humans and animals should be emphasised. Owners should be aware of the fact that not all human edible foods are safe to be consumed by their pets. Thus, care should be taken in food preparation, in order to prevent accidental ingestion by pets.
> Acknowledgements
Authors would like to thank professor Athanasios Dinopoulos, DVM, PhD, Laboratory of Anatomy, Histology & Embryology, School of Veterinary Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, for his contribution to mushroom identification.
> Referenses
1. Spoerke D. Mushrooms. In: Small Animal Toxicology. Peterson ME, Talcott PA (eds). 2nd edn. WB Saunders Co: Philadelphia, 2006, pp. 860-884.
2. Puschner B, Wegenast C. Mushroom Poisoning Cases in Dogs and Cats: Diagnosis and Treatment of Hepatotoxic, Neurotoxic, Gastroenterotoxic, Nephrotoxic and Muscarinic Mushrooms. Vet Clin North Am Small Anim Pract 2012, 42: 375-387.
3. Puschner B. Mushrooms. In: Small Animal Toxicology. Peterson ME, Talcott PA (eds). 3rd edn. Elsevier Saunders: Missouri, 2013, pp. 659-676.
4. Brownie C. Poisonous mushrooms. 2006, http://www. merckvetmanual.com/toxicology/poisonous-mushrooms/overview-ofpoisonous- mushrooms, (accessed 14 January 2017).
5. Beug M, Shaw M, Cochran K. Thirty-plus years of mushroom poisonings: summary of the approximately 2,000 reports in the NAMA Case Registry. McIlvainea 2006, 16(2): 47-68.
6. Beug M. 2008 NAMA Toxicology Committee report: North American Mushroom Poisonings. McIlvainea 2009, 18: 45-54.
7. Beug M. 2013 NAMA Toxicology Committee report: North American Mushroom Poisonings. McIlvainea 2014, 24: 1-13.
8. Beug M.2014 NAMA Toxicology Committee report: North American Mushroom Poisonings. McIlvainea 2015, 25: 1-16.
9. Veterinary Poisons Information Service (VPIS). VPIS Annual Report 2014. 2014, https://vpisglobal.com/our-research/, (accessed 20 January 2017).
10. Bates N, Edwards N, Dentiger B, Ainsworth A. Fungal ingestion in companion animals. Vet Rec 2014, 175: 179-180.
11. Ridgway R. Mushroom (Amanita pantherina) poisoning. J Am Vet Med Assoc 1978, 172: 681-682.
12. Herreria-Bustillo VJ, Saiz-Alvarez R, Jasani S. Suspected muscarinic mushroom intoxication in a cat. J Feline Med Surg 2012, 15(2): 160-162.
13. Tokarz D, Poppenga R, Kaae J, Filigenzi M, Lowenstine LJ, Pesavento P. Amanitin Toxicosis in Two Cats with Acute Hepatic and Renal Failure. Vet Path 2012, 49(6): 1032-1035.
14. Palm C, Kanakubo K. Blood purification for intoxications and drug overdose. In: Small Animal Critical Care Medicine. Silverstein D, Hopper K (eds). 2nd edn. Elsevier Saunders: Missouri, 2005, pp. 390-394.
15. Cope RB. Toxicology Brief: Mushroom poisoning in dogs. Vet Med 2007, Feb: 95-100.
16. Turner N, Szczawinski A. Common poisonous plants and mushrooms of North America. 1st edn. Timber Press: Portland (OR), 1991.
17. Benjamin DR. Mushrooms: poisons and panaceas. 1stedn. WH Freeman & Co: New York, 1995.
18. Torricelli R, Johansson S, Wuthrich B. Ingestive and inhalative allergy to the mushroom Boletus edulis. Allergy 1997, 52: 747-751.
19. Roncarolo D, Minale P, Mistrello G, et al. Food allergy to Boletus edulis. J Allergy Clin Immunol 1998, 101: 850-851.
20. Beug M. Mushroom Poisoning in North America: Summary of Voluntary Reporting and News Articles for 2015 and 2016. McIlvainea 2017, 26.
21. Armes, IA. Mushroom poisoning syndromes. 2017, www.namyco. org/mushroom_poisoning_syndromes.php, (accessed 24 January 2017).
22. Butera R, Locatelli C, Coccini T, Manzo L. Diagnostic accuracy of urinary amanitin in suspected mushroom poisoning: a pilot study. J Toxicol Clin Toxicol 2004, 42 (6): 901-912.
23. Jehl F, Gallion C, Birckel A, Jaeger A, Flesch F, Minck R. Determination of α-amanitin and β-amanitin in human biological fluids by highperformance liquid chromatography. Analyt Biochem 1985, 149 (1): 35-42.
24. Faulstich H, Talas A, Wellhoner H. Toxicokinetic of labeled amatoxins in the dog. Arch Toxicol 1985, 56: 190-194.
25. Beug M. Amatoxin Mushroom Poisoning in North America 2015- 2016. McIlvainea 2017, 26.



