> Abstract
The etiopathogenetic relationship between the intestinal flora and the presence of bacteria in bile in feline gastrointestinal disorders has not been studied previously. The aim of the study was the bacteriological analysis of duodenal juice and bile in cats with chronic inflammatory bowel disease (IBD), cholangitis, pancreatitis, and their combinations (triaditis), as well as in cats with intestinal lymphoma. In this prospective study 49 sick cats were included, 45 (25 symptomatic, 20 asymptomatic) with histopathological evidence of IBD, and/or cholangitis, and/or pancreatitis and four with intestinal lymphoma, as well as eight healthy cats. Samples of duodenal juice and bile were collected during exploratory laparotomy and cultured under aerobic, anaerobic and microaerobic conditions in order to isolate, enumerate and identify bacteria following standard microbiological guidelines. Comparisons of the bacterial populations of the duodenum among the groups of cats of the study regarding the growth of aerobic (P=0,831), anaerobic (P=0,406) and the total population of bacteria (P=0,752) did not outline any statistically significant differences. A statistically significant difference was noted in cats with triaditis regarding the growth of anaerobic Clostridium spp. (P=0,055). Τhe bile samples of the normal and most (48/49, 98%) of the sick cats were bacteriologically negative. However, growth of a strain of Enterobacter cloacae was noted in a bile sample of a cat with IBD and pancreatitis. Inflammatory disorders of the small intestine, the liver, and the pancreas are not related to bacterial growth in the bile. In order to confirm the possibility of triaditis being correlated with an overgrowth of anaerobic intestinal, such as Clostridium spp., further research using sensitive molecular diagnostics will be necessary.
> Introduction
Canine and feline intestinal flora is composed of several hundreds to thousands of species of aerobic, microaerophilic and obligate anaerobic bacteria, the composition of which is specific and characteristic to each animal with differences even between individuals of the same species. However, its basic composition has not been fully revealed. 1 According to studies based on bacterial culturing,2,3,4 the main species of bacteria prevalent in the feline small intestine are Escherichia coli and strains of the genera Bacteroides, Lactobacillus, Streptococcus, Enterococcus, Staphylococcus and Clostridium, in various percentages depending on the segment of the gastrointestinal tract and its distance from the large intestine. In the stomach bacterial populations ranging from 101 to 106 cfu/g have been reported, whereas in the duodenum and the jejunum bacterial populations from 105 up to 109 cfu/ml have been noted in some cats. The number and range of bacterial strains increases in the ileum (107 cfu/ ml) and even more in the colon (>109 cfu/ml).2,14 Αerobes are detected in higher number in the cranial segments of the intestinal tract, whereas anaerobes predominate in the colon. In cats, however, the number of anaerobic bacteria colonising the small intestine appears to be higher than in dogs.2,4,5
In recent years it has been proven that, similar to humans, in dogs and cats, alterations in the composition of intestinal flora are implicated in chronic enteropathies.6-13 Higher than normal colonisation of enteric bacteria in the proximal segment of the small intestine characterise the bacterial overgrowth syndrome, which is involved in the development of chronic gastrointestinal signs. The standard diagnostic procedure includes culturing intestinal juice collected from the duodenal lumen under aerobic and anaerobic conditions.14,15 Bacterial overgrowth in the cat is defined as an increase in the bacterial population of the proximal small intestine higher than 1,1x109 cfu/ml of intestinal content.4,14,15 In healthy cats the total bacterial population in the proximal small intestine shows high variation and it may usually surpass the numbers initially set as bacterial overgrowth. Moreover, apart from alterations in the number of bacteria, changes in the bacterial species comprising the intestinal flora are also of great significance. This disorder is described by the term “intestinal dysbiosis”.5,15
Alterations in the composition of intestinal flora in the proximal small intestine, and the presence of bacteria in bile and their relationship with the etiopathogesis of inflammatory disorders of the feline gastrointestinal tract still remain unclear. The aim of the present study was to reveal the duodenal bacterial composition and the presence of bacteria in the bile of cats with IBD, cholangitis, pancreatitis, or combinations of the aforementioned, including the clinical syndrome of triaditis (IBD, cholangitis and pancreatitis), or with intestinal lymphoma, and to compare all of the above with normal cats. The present study exists in continuation of a previous research project about the clinical, laboratory and histopathologic presentation of the feline triaditis complex,16 in order to investigate the etiopathogenesis of inflammatory bowel disorders.
> Materials and methods
- Study design
This prospective study involved domestic cats, which were examined in the Department of Internal Medicine of the Companion Animal Clinic, School of Veterinary Medicine, A.U.Th. (February 2008 - February 2011). Τhe study protocol was approved by the Department of the School of Veterinary Medicine (Clinical research ethical approval: Special General Assembly of the Department of Veterinary Medicine no. 430/20-11-2007) and by the appropriate National Department (Approval of clinical research: Veterinary Administration Office of Thessaloniki, protocol no. 13/3657/29.03.2010). No procedure was undertaken in the cats of the study without signed owner consent. For study purposes, two categories of cats were examined: symptomatic cats, presented to the Companion Animal Clinic with chronic clinical signs, which could be attributed to inflammatory bowel disease, including triaditis or intestinal lymphoma (in particular they had persistent or recurrent one or some combination of the following clinical signs: depression, increased or decreased appetite, vomiting, fecal consistency abnormalities, jaundice, weight loss), as well as asymptomatic cats presented for ovariohysterectomy. At least two weeks prior to diagnostics, all of the clinically healthy cats were admitted to a separate area of the hospitalisation ward of the Department of Internal Medicine of the Companion Animal Clinic in order to adapt to their surroundings, be fed exclusively with commercial high quality dry food (base components: 33.8-34.2% protein, 21.9-22.3% fat, 36.9-38.1% carbohydrates, 1.1-1.3%, fibre and 0.80-0.88% calcium in dry matter) (Feline Adult Optimal Care™ Chicken-Dry, Science Plan™, Hill’s™) to be monitored and diagnostic tests to be performed.
The selection of cats for the study was made based on the following inclusion criteria: (1) age over one year of both genders and of various breeds, (2) diet with commercial cat food (dry and/or canned) for at least eight weeks prior to the initial physical examination, (3) written owner consent for the exploratory laparotomy, biopsy sampling and collection of biological materials, (4) histopathological evidence of inflammation (enteritis, cholangitis, pancreatitis, or a combination of the above) or intestinal lymphoma with or without compatible clinical signs at the time of physical examination (study group) or normal clinical and histopathological findings (control group).
Exclusion criteria were as follows: (1) presence of clinical or laboratory findings of other pathological conditions, which could affect the liver, the pancreas, or the small intestine, (2) presence of histopathological findings in the liver, the pancreas and the small intestine other than those investigated for the purposes of the study, (3) positive results in fecal parasitological analysis, (4) positive results in serological detection of antibodies against feline immunodeficiency virus (FIV), antigen of feline leukemia virus (FeLV) and antibody against feline coronavirus (FCoV) and feline infectious peritonitis (FIP), (5) abnormal results of total or free thyroxin concentrations in blood serum (T4, Free T4), (6) administration of drugs such as antimicrobials, anti-inflammatories, or immunosuppressants, in the last two weeks prior to admission.
Symptomatic cats: During the study, 302 cats with clinical signs were evaluated, 82 of which fulfilled the inclusion criteria. Based on owner consent for biopsy sampling, 39 cats were fully investigated, 25 of which were included in the study based on the predetermined inclusion criteria.
Αsymptomatic cats: During the same time period, 39 cats without clinical signs were fully investigated, following the same diagnostic protocol with symptomatic cats. Following histopathology results, eleven cats were excluded through the predetermined criteria, eight were found to be normal, whereas in twenty cats histopathological evidence of inflammation was uncovered in the organs under investigation.
Following histopathological results all cats with inflammatory lesions in the intestine, the liver, and the pancreas regardless of the presence of clinical signs at the time of sampling, as well as cats with small intestinal lymphoma, were included in the study group of cats with abnormal findings intended for diagnostic investigation. Asymptomatic cats, with normal histopathological findings in the liver, pancreas, and the intestine constituted the control group.
Thus, in our study, 57 cats were included in total: 49 cats with abnormal findings (45 cats with histopathologicaly evidence of various combinations of IBD, cholangitis, pancreatitis, 4 with intestinal lymphoma) and 8 cats as normal controls.
- Groups of cats
Based on histopathological results, the cats of the study were classified into eight groups, which are presented in Table 1. In one cat with cholangitis, one cat with IBD and cholangitis, two cats with simultaneous IBD, cholangitis and pancreatitis, two cats with IBD and pancreatitis, three cats with IBD and a cat with intestinal lymphoma,duodenal juice was not sampled for analysis.
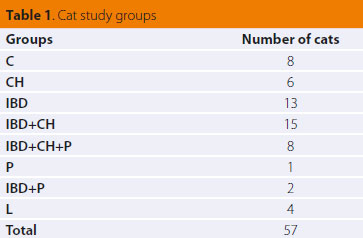 |
C: controls
CH: cats with histopathological evidence of cholangitis
IBD: cats with histopathological evidence of chronic inflammatory bowel disease
IBD+CH: cats with histopathological evidence of chronic inflammatory bowel disease and cholangitis
IBD+CH+P: cats with histopathological evidence of chronic inflammatory bowel disease, cholangitis and pancreatitis
P: cats with histopathological evidence of pancreatitis
IBD+P: cats with histopathological evidence of chronic inflammatory bowel disease and pancreatitis
L: cats with histopathological evidence of small intestinal lymphoma |
- Histopathological characteristics of study groups
IBD: In total, thirty eight cats had histopathological evidence of the lymphocytic/plasmacytic type of IBD. In all of the latter there was infiltration of lymphocytes, plasmacytes and macrophages in the intestinal mucosa, whereas neutrophils were observed in variable numbers and more rarely, occasional eosinophils. The infiltrations, combined with architectural lesions in the intestinal epithelium, extended in all entire parts (duodenum, jejunum, ileum) with a variable degree of severity.
Cholangitis: Twenty nine cats had histopathological evidence of cholangitis. The of majority lesions was defined by infiltration of portal areas consisting primarily of lymphocytes and to a lesser extent by plasmacells, fibrosis, and hyperplasia of the bile ducts (lymphocytic type of cholangitis). In a small number of cats (5/29), in addition to mononuclear cells neutrophils were present (chronic neutrophilic cholangitis).
Pancreatitis: Eleven cats showed lesions of chronic pancreatitis, characterised by mononuclear cell infiltration and fibrosis. In five of them, there was also a considerable number of neutrophils (three cases were classified as chronic-active pancreatitis and the other two, where necrotic lesions were present, as acute necrotic pancreatitis in combination with chronic pancreatitis).
Lymphoma: Four cats had histopathological findings compatible with intestinal lymphoma. The latter constituted of diffuse aggregations of a uniform population of lymphocytes in the connective tissue and submucosal layer with multifocal infiltrations of the muscularis, whereas in some cases they were also present in the innermost mucosa. Very small numbers of the other types of inflammatory cells were observed, whereas micro-erosions and erosions of the epithelial surface were noted. In one cat an increased number of neutrophils was noted in the connective tissue layer.
- Physical and Diagnostic examinations
In all 57 cats included in the study the history was initially obtained and a general physical examination was performed. Diagnostic procedures ((<3 days prior to exploratory laparotomy) for the 57 cats included fecal parasitological examination and the detection of Giardia spp. antigen, standard urinalysis, complete blood count, biochemistry in blood serum including: albumin (ALB), blood urea nitrogen (BUN), creatinine (CREA), alkaline phosphatase (ALP), alanine aminotransferase activity (ALT), g-glutamine transferase activity (γGT), aspartate aminotransferase activity (AST), total bilirubin (TBIL), lipase activity, ionised calcium (Ca), phosphorus (P), potassium (Κ), sodium (Na), blood coagulation profile including prothrombin time (PT) and partial thromboplastin time (PTT) (52/57), serum total thyroxin (Τ4) and free thyroxin (Free T4), serum feline immunoreactivity of pancreatic lipase (fPLI measured by Spec fPL®)17 (56/57) trypsin like immunoreactity (fTLI) and testing for viral origin disorders including FIV, FeLV, and coronavirus for FIP. Diagnostic imaging included thoracic and abdominal radiographs, (49/57) and abdominal ultrasonography (56/57). Full thickness biopsy samples were collected for histopathological diagnosis (at least five from each cat: one from the liver, the pancreas, the duodenum, the jejunum, and the ileum) via exploratory laparotomy and were evaluated in a blinded fashion by a specialised veterinary pathologist (P. T.), based on internationally acceptable histopathological criteria.18-20
- Intestinal content and bile sampling
Prior to intestinal biopsy, a sample of duodenal juice was obtained from the duodenum. To this purpose, the duodenum was located and by “massaging” its full length any contents present were collected in the middle segment. Duodenal juice was removed by suction through plastic intravenous catheter (Abbocath-T I.V. Catheter 20 G x 1,25”, VenisystemsTM, Abbott, Ireland) attached on a 20 ml syringe.15 Τhe sample of duodenal juice was immediately placed in a sterile, glass vacuum blood collection vial without anticoagulant.
During exploratory laparotomy, 1 ml of bile was obtained from the gall bladder, by use of sterile 1 ml syringe and a 25 G needle. In cases when bile could not be aspirated (e.g. increased viscosity), a wider bore needle was used (23-21 G). The samples were immediately transfused to a sterile vacuum glass vial for blood collection, without anticoagulant (Venoject®, Terumo Europe N.V., Leuven, Belgium).
Vials containing bile and intestinal content were immediately placed in a transportation refrigerator (temperature levels of 4-6 °C) and within one hour from sampling they were transported to the laboratory of microbiology, where they were inoculated in the appropriate growth mediums under specific aerobic, microaerophilic and anaerobic conditions, aiming in growth of any bacteria in the samples.
- Culturing of duodenal juice and bile
Isolation and enumeration of bacteria
For the isolation and enumeration, as well as the identification of bacteria standard microbiological guidelines were employed.21 For the isolation of aerobic and facultative anaerobic bacteria, such as Enterobacteriaceae spp., Lactobacillus spp., Staphylococcus spp., Streptococcus spp., Enterococcus spp. and Pseudomonas spp., the mediums Blood Agar, MacConkey Agar, Rogosa Agar and Bile Esculin Agar were used. For the isolation of anaerobic bacteria, such as Clostridium spp., Bacteroides spp., Peptostreptococcus spp. and Eubacterium spp., the following mediums were used: Anaerobic Agar acc. to Brewer and TSC-Agar (Tryptose Sulfite Cycloserine Agar, Perfringens Agar). For the isolation of microaerophilic bacterial strains, such as Campylobacter spp., the special medium Campylobacter Selective Agar was used.
In order to calculate the bacterial population of samples, the technique of serial dilutions (10-1 up to 10-6) and inoculation of every dilution in agar plates with the spread plate method was used. From each dilution usually two agar plates were inoculated for every growth medium. Agar plates were incubated in 37°C in aerobic, anaerobic and microaerophilic conditions, depending on the inoculated substrate. For incubation in anaerobic conditions specialised containers were used (GasPakTM EZ Anaerobe Pouch System, Anaerobe Gas Generating pouch system with indicator, Becton Dickinson, NJ, USA), as well as for incubation of microaerophilic bacteria (GasPakTM Campy Pouch System, BBLTM Microaerophilic Campy pouch system, Becton Dickinson, NJ, USA). Τhe agar plates were daily monitored for the presence of bacterial growth up to 48 hours for aerobic cultures and up to six days for anaerobic and microaerophilic cultures.
In order to calculate the population of bacteria, the colonies that were observed in aerobic, anaerobic and microaerophilic conditions were counted, including the agar plates containing 30-300 colonies. The total of visible colonies from both agar plates that had been inoculated from each dilution and the median of both agar plates were calculated. Finally, the number of bacteria capable of forming colonies was expressed per 1 ml of initial sample (colony forming units, cfu/ml).
Bacterial identification
For the identification of bacterial strains the following were evaluated: (1) growth in special media for isolation and identification of bacteria [(i) Bile Esculin agar (BBLTM Bile Esculin Agar, Becton Dickinson, Maryland, USA): for isolation of Enterococcus spp. and differentiation from Streptococcus spp. (ii) Campylobacter Selective agar: for isolation of Campylobacter spp. (iii) TSC agar: for isolation of Clostridium spp. (iv) Rogosa agar: for isolation of Lactobacilli (v) Anaerobic agar acc. to Brewer: for isolation of Clostridium spp. and other anaerobic or microaerophilic bacteria.] (2) the morphological characteristics of bacteria post staining (Gram stain). (3) the initial biochemical characteristics: catalase testing, oxidase testing, positive or negative growth in MacConkey agar, indole testing, nitric salt induction, production of lecithinase, production of lipase. (4) sensitivity or not to the antimicrobial substance vancomycin, as an additional test beyond Gram staining, for the differentiation between Gram positive and Gram negative bacteria. (5) For specific identification of Enterobacteriaceae spp. the API 20 E system was used (API® bio- Mérieux Inc., Durham NC, USA).
> Statistical analysis
The cats of the study were classified into groups using histopathological diagnosis as a criterion. Data processesing and comparisons were made among study groups. Cats with pancreatitis alone, with IBD lesions in combination with pancreatitis and cats with intestinal lymphoma were excluded from statistical analysis, due to small sample size. For the synoptic presentation of statistical results absolute and relative frequencies (percentages %), measures of central tendency (mean, median) and measures of spread-dispersion [(minimum (min)-maximum (max) values and standard deviation] were calculated. For comparisons of means and medians the Kruskal-Wallis and Mann-Whitney tests were employed. For comparisons of proportions (percentages %) z-test was used with Bonferroni correction to the significance level. In all statistical analyses the observed significance level (P-value) was estimated, as appropriate, either with the Exact Method, or a Monte-Carlo simulation based on 10.000 resampling cycles. 22 Τhe level of statistical significance was set at α=0,05 (P≤0,05). Statistical analyses were performed by the IBM SPSS v. 20.0 statistical package (USA, Chicago: Illinois) with the Exact Tests subsystem installed. (Statistical pack IBM SPSS v.20.0)
> Results
Duodenal bacterial population
Aerobic and anaerobic bacterial species isolated during culture of duodenal juice per group of cats, are described in Tables 2 and 3. No growth of microaerophilic bacteria of the genus Campylobacter spp. was observed in any of the duodenal juice cultures from the cats in this study.
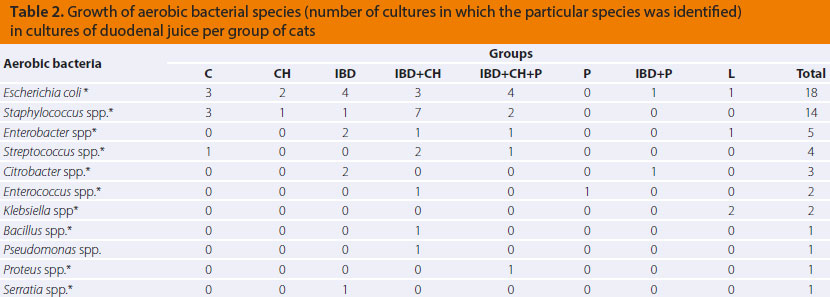
* facultative anaerobes
C: controls (n=8)
CH: cats with histopathological evidence of cholangitis (n=5)
IBD: cats with histopathological evidence of chronic inflammatory bowel disease (n=10)
IBD+CH: cats with histopathological evidence of chronic inflammatory bowel disease and cholangitis (n=14)
IBD+CH+P: cats with histopathological evidence of chronic inflammatory bowel disease, cholangitis and pancreatitis (n=6)
P: cats with histopathological evidence of pancreatitis (n=1)
IBD+P: cats with histopathological evidence of chronic inflammatory bowel disease and pancreatitis (n=1)
L: cats with histopathological evidence of small intestinal lymphoma (n=3)
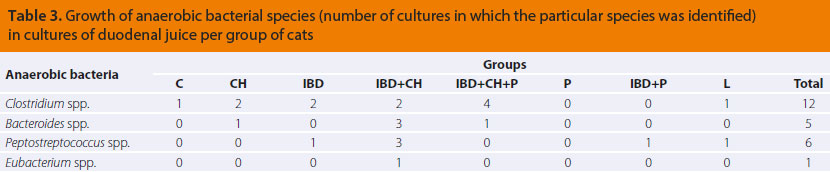
C: controls (n=8)
CH: cats with histopathological evidence of cholangitis (n=5)
IBD: cats with histopathological evidence of chronic inflammatory bowel disease (n=10)
IBD+CH: cats with histopathological evidence of chronic inflammatory bowel disease and cholangitis (n=14)
IBD+CH+P: cats with histopathological evidence of chronic inflammatory bowel disease, cholangitis and pancreatitis (n=6)
P: cats with histopathological evidence of pancreatitis (n=1)
IBD+P: cats with histopathological evidence of chronic inflammatory bowel disease and pancreatitis (n=1)
L: cats with histopathological evidence of small intestinal lymphoma (n=3)
The numerical estimation of the total of aerobes, anaerobes as well as the entire bacterial population from cultures of duodenal juice of cats in this study are reported per group in Table 4.
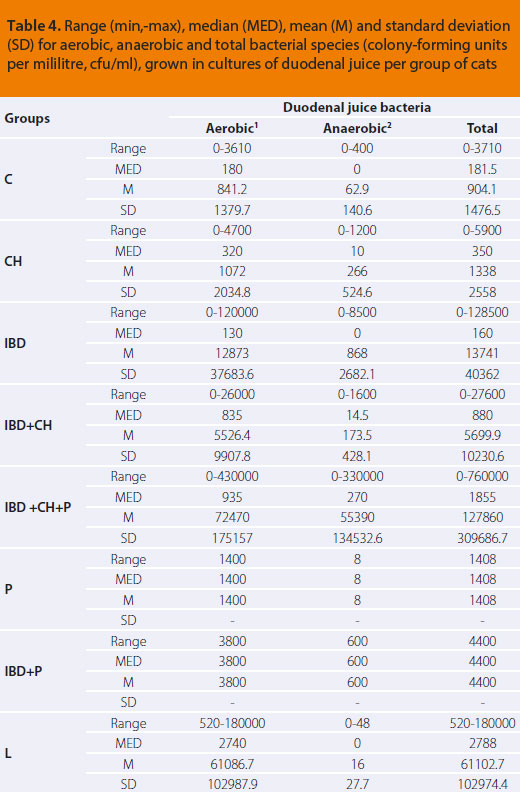 |
1aerobic and facultative anaerobic bacteria
2strictly anaerobic bacteria
C: controls (n=8)
CH: cats with histopathological evidence of cholangitis (n=5)
IBD: cats with histopathological evidence of chronic inflammatory bowel disease (n=10)
IBD+CH: cats with histopathological evidence of chronic inflammatory bowel disease and cholangitis (n=14)
IBD+CH+P: cats with histopathological evidence of chronic inflammatory bowel disease, cholangitis and pancreatitis (n=6)
P: cats with histopathological evidence of pancreatitis (n=1)
IBD+P: cats with histopathological evidence of chronic inflammatory bowel disease and pancreatitis (n=1)
L: cats with histopathological evidence of small intestinal lymphoma (n=3) |
Comparison of the bacterial population of the duodenum between the feline study groups regarding the growth of aerobics (P=0,831), anaerobics (P=0,406) and the total bacterial population (P=0,752) did not reveal statistically significant differences.
The numerical estimation of the most common aerobic and anaerobic bacterial growth in cultures of duodenal juice of cats in this study is presented per group in Table 5.
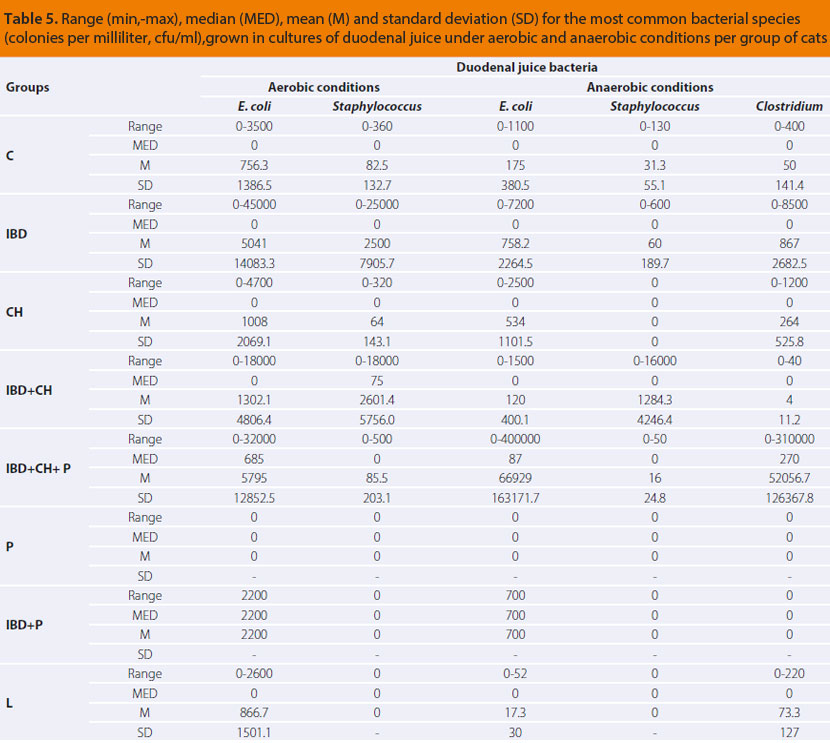
C: controls (n=8)
CH: cats with histopathological evidence of cholangitis (n=5)
IBD: cats with histopathological evidence of chronic inflammatory bowel disease (n=10)
IBD+CH: cats with histopathological evidence of chronic inflammatory bowel disease and cholangitis (n=14)
IBD+CH+P: cats with histopathological evidence of chronic inflammatory bowel disease, cholangitis and pancreatitis (n=6)
P: cats with histopathological evidence of pancreatitis (n=1)
IBD+P: cats with histopathological evidence of chronic inflammatory bowel disease and pancreatitis (n=1)
L: cats with histopathological evidence of small intestinal lymphoma (n=3)
Comparisons of the duodenal bacterial population of the cat study groups, regarding the growth of Εscherichia coli, which was evaluated in aerobic (P=0,317) as well as anaerobic conditions (P=0,313), and Staphylococcus spp., in both aerobic (P=0,332) and anaerobic conditions (P=0,279), did not show any statistically significant differences among groups. Statistically significant differences were revealed during comparison of results among groups in regard to growth of anaerobic Clostridium spp., as they are presented in Table 6.
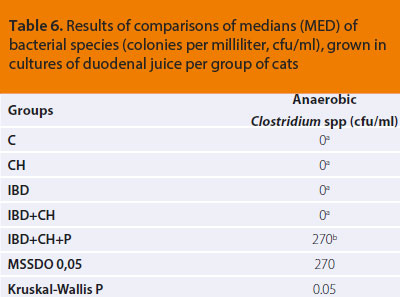 ΜSSDO: Minimum Statistically Significant Difference Observed at a significance level of P=0,05
ΜSSDO: Minimum Statistically Significant Difference Observed at a significance level of P=0,05
a, b: In the same column of the table medians followed by common letter (superscript) do not differ significantly according to the results of a series of Mann-Whitney tests. A statistically significant difference exists between medians with different superscript letter.
C: controls (n=8)
CH: cats with histopathological evidence of cholangitis (n=5)
IBD: cats with histopathological evidence of chronic inflammatory bowel disease (n=10)
IBD+CH: cats with histopathological evidence of chronic inflammatory bowel disease and cholangitis (n=14)
IBD+CH+P: cats with histopathological evidence of chronic inflammatory bowel disease, cholangitis and pancreatitis (n=6)
Presence of bacteria in bile
Bile cultures of the control group were negative for bacterial growth. From the cat groups with abnormal findings, only one bile culture was positive from the IBD+P group, in which Enterobacter cloacae was isolated (Table 7).
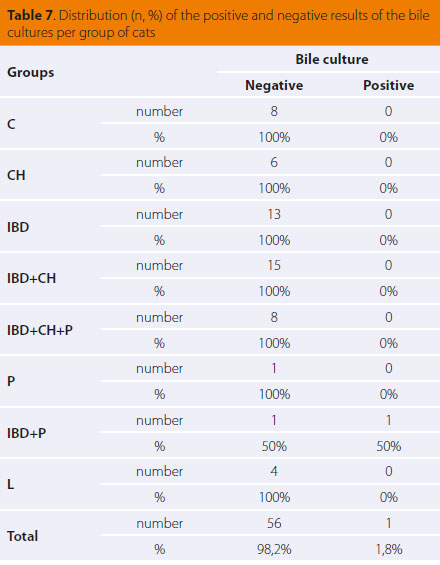
C: controls (n=8)
CH: cats with histopathological evidence of cholangitis (n=6)
IBD: cats with histopathological evidence of chronic inflammatory bowel disease (n=13)
IBD+CH: cats with histopathological evidence of chronic inflammatory bowel disease and cholangitis (n=15)
IBD+CH+P: cats with histopathological evidence of chronic inflammatory bowel disease, cholangitis and pancreatitis (n=8)
P: cats with histopathological evidence of pancreatitis (n=1)
IBD+P: cats with histopathological evidence of chronic inflammatory bowel disease and pancreatitis (n=2)
L: cats with histopathological evidence of small intestinal lymphoma (n=4)
> Discussion
There was wide variability in the duodenal bacterial populations among the cat groups. However, comparisons did not reveal statistically significant differences in the total bacterial populations, as well as in the subgroups of aerobic and anaerobic duodenal bacteria between controls (C group) and cats with abnormal findings of all groups (IBD, Ch, IBD+Ch, IBD+Ch+P, Tables 4, 5 & 6). By reviewing our findings and in comparison to the referred as the feline normal intestinal flora, intestinal bacterial overgrowth was not substantiated in any of the cats in our study. In the control group the small intestinal bacterial population ranged from 0 to 3,7x103 cfu/ ml (mean 9x102). Among the groups of sick cats, the most numerous bacterial populations were noted in the triaditis group (IBD+Ch+P, Table 4), ranging from 0 to 7,6x105 cfu/ml (mean 1,2x105).
Both the mean and maximum values of bacteria observed in all study groups were found to be within the previously published reference range for the normal feline bacterial flora (105-108 cfu/ ml).2,14,15 However, in our study a predominance of anaerobic species of the genus Clostridium was observed in cats of the triaditis group (IBD+Ch+P, Table 5) compared to the rest of the study groups. Clostridium spp. (division Firmicutes, family Clostridiaceae, including at least 70 different species) constitute most of the cecal flora, however, they can be detected in other intestinal segments performing different functions.5 They are therefore part of the normal feline small intestinal flora despite their anaerobic nature.2,15
The etiopathogenetic connection between abnormal variations in the intestinal flora and induction of inflammation has not yet been clarified.1 An increase in several bacterial strains of Proteobacteria, such as Escherichia coli, and a reduction the Firmicutes and especially of the diversity of certain Clostridium spp. have both been reported as common disorders.1,10-13 In dogs with IBD a reduction in the diversity of small intestinal bacterial flora has been observed.23 Research in cats with IBD has proven the existence of «intestinal dysbiosis» in sick cats, with the Enterobacteriaceae spp., Clostridium spp., Bacteroides spp. and Streptococcus spp. corresponding to 91% of bacteria attached to the intestinal mucosa and Εscherichia coli comprising 30% of the Enterobacteriaceae spp..8 A different study indicated that strains from the genus Desulfovibrio predominated in the intestinal flora of cats with IBD, whereas strains of the Bifidobacterium and Bacteroides genera predominated in the bacterial populations of healthy cats.7 In our study, at first, it seemed like a paradox that even though cats of the triaditis group (IBD+CH+P, Table 5) had increased populations of Clostridium spp. in the duodenum, similar increase was not observed in the rest of the groups. Τhis fact underlines the complicated nature of the etiopathogenetic connection between the three pathological conditions, as well as “chronologically placing” their coexistence from an evolutionary perspective in more advanced stages compared to their combinations in pairs. In the future, sensitive molecular methods could give answers, regarding the increased populations of Clostridium spp. evidenced in our study, diversity and their contribution to the pathogenesis of triaditis.
The microbiological analysis of bile, concerning the diagnosis of cholangitis, usually includes culture in aerobic and anaerobic conditions as well as antibiotic sensitivity testing in order to indicate the proper therapeutic regimen.24-27 Culturing bile is preferred to culturing liver biopsy samples or gall bladder wall samples, because of improved rates of microorganism detection.28 Despite the ruling hypothesis that bile in healthy cats is microbiologically sterile,25,29 some researchers claim that bacterial translocation from duodenum to bile can occur in healthy as well.28 In our study, however, no bacterial growth was noted in bile cultures from healthy controls.
Regarding the types of feline cholangitis, in the acute neutrophilic cholangitis, isolation of mostly Enterobacteriaceae spp. originating from the duodenal flora in the bile is a common occurrence and confirms the diagnosis.24,25,28,30-35 Bacterial translocation from the intestinal tract to the gall bladder can occur either through reflux of bile from the duodenum, or through the hematogenous or lymphic routes.25 It is maintained that inflammatory bowel disease and pancreatitis can predispose to cholestasis, resulting in reflux of pancreatic secretions and/or bacteria towards the liver.32,36 In chronic neutrophilic cholangitis, bile culture is negative in most cases. It is theorised that this occurs due to i) either the bacteriostatic properties of bile, ii) or because the initial bacterial invasion was restricted by the immune system, iii) or due to previous use of antimicrobials, and iv) in cases when bacteria are not the immediate cause of the inflammatory disorder.24,37-39 However, even in chronic cholangitis of non-bacterial origin, chronic infiltration of bile ducts by inflammatory cells results in a risk for secondary hepatic infection by Enterobacteriaceae spp., such as Εscherichia coli.26
The lymphocytic type of cholangitis appears to originate from an immune-mediated aetiopathogenetic mechanism.40-42 However, there is also a theory that this particular type of cholangitis represents the chronic stage of acute neutrophilic cholangitis or an ascending (originating from the duodenum) bacterial infection.31,37,38 Τhere is only a small amount of data on which the hypothesis of a primary bacterial infection can be based.42 Two studies have been published concerning a small group of cats with cholangitis/cholangiohepatitis in which bacterial DNA of the Helicobacter genus has been detected, although the pathophysiological significance of this finding has yet to be clarified.43,44 It is worthy of note that until the present day, there is no evidence to support the involvement of Helicobacter spp. to IBD and pancreatitis in cats.8,45 Furthermore, in an experimental study, moderate inflammation in zone 1 of the feline liver was caused after infection with Bartonella spp.46 Even though there is considerable evidence of an immune-mediated mechanism causing cholangitis, the actual etiopathogenesis of the disorder remains a mystery.42
Τhe results of our study do not support the hypothesis of a primary microbial infection, considering that all the bile samples from cats with cholangitis were found to be bacteriologically sterile. From cats lacking histopathological evidence of cholangitis, only a single bile sample was found positive with growth of the bacterium Εnterobacter cloacae. This was a cat with pancreatitis and IBD (Table 7, IBD+P group) together with cholestasis, without histopathological evidence of cholangitis or feline hepatic lipidosis. As previously mentioned, in this particular case cholestasis, as a result of obstruction in bile flow due to pancreatitis, became a risk factor for the translocation of Εnterobacter cloacae, which is part of the normal small intestinal flora, toward the gall bladder. Unfortunately, culture of duodenal content was not performed on this cat; therefore its intestinal flora is unknown. Such a hypothesis, however, cannot explain the inflammation in the bile duct system observed in 29 cats with histopathological evidence of cholangitis in our study, in which bile cultures were negative. The possibility of bacterial translocation toward the liver and consequently the pancreas via the common bile and pancreatic duct, does not exclude the theory of an immune response to such bacterial invasion.
To summarise, the present study indicated that inflammatory disorders of the gastrointestinal tract relating to triaditis as well as intestinal lymphoma do not seem to be pathogenetically related to the bacterial flora of the duodenum or any presence of bacteria in bile. The intestine and the liver play a particularly significant role in immunity. This complicated system of the intestinal flora may affect several functions as well as the global health and every disruption in its interactions with the local intestinal immune mechanisms could lead to gastrointestinal disease.1,6-10 In conclusion, an immune-mediated mechanism could be involved in the development of triaditis in cats.42,47 Modern molecular methods of analysis are expected to give more answers in the investigation of any correlations between intestinal flora and its variability with histopathological lesions of inflammation in the intestine, liver and pancreas.
> Acknlowledgements
The authors would like to acknowledge the contribution of Dr. G. Menexes, Assistant Professor of Biometrics and Agricultural Experimentation, Faculty of Agriculture, Forestry and Natural Environment, School of Agriculture, Α.U.Th. for performing the statistical analysis of the results of this study. They would also like to thank the cat owners, veterinarians, students and the staff of the Companion Animal Clinic that became a part of this research project.
The first author (F.F.) was supported by the State Scholarships Foundation (code no 5321) in order to realise part of the present study.
> References
1. Suchodolski JS. Companion Animals Symposium: Microbes and gastrointestinal health of dogs and cats. J Anim Sci 2011, 89: 1520-1530.
2. Johnston K, Lamport A, Batt RM. An Unexpected Bacterial- Flora in the Proximal Small-Intestine of normal cats. Vet Rec 1993, 132: 362-363.
3. Sparkes AH, Papasouliotis K, Sunvold G, Werrett G, Clarke C, Jones M, Gruffydd-Jones TJ, Reinhart G. Bacterial flora in the duodenum of healthy cats, and effect of dietary supplementation with fructo-oligosaccharides. Am J Vet Res 1998, 59: 431-435.
4. Johnston KL, Swift NC, Forster-van Hijfte M, Rutgers HC, Lamport A, Ballevre O, Batt. RM. Comparisοn of the bacterial flora of the duodenum in healthy cats and cats with signs of gastrointestinal tract disease. J Am Vet Med Assoc 2001, 218: 48–51.
5. Suchodolski JS. Gastrointestinal Microbiota. In: Canine and Feline Gastroenterology. Washabau RJ and Day MJ (eds). Elsevier Saunders: Missouri, 2013, pp. 32-41.
6. German AJ, Day MJ, Ruaux CG, Steiner JM, Williams DA, Hall EJ. Comparison of direct and indirect tests for small intestinal bacterial overgrowth and antibiotic-responsive diarrhea in dogs. J Vet Intern Med 2003, 17: 33-43.
7. Inness VL, McCartney AL, Khoo C, Gross KL, Gibson GR. Molecular characterisation of the gut microflora of healthy and inflammatory bowel disease cats using fluorescence in situ hybridisation with special reference to Desulfovibrio spp. J Anim Physiol Anim Nutr (Berl) 2007, 91: 48-53.
8. Janeczko S, Atwater D, Bogel E, Greiter-Wilke A, Gerold A, Baumgart M, Bender H, McDonough PL, McDonough SP, Goldstein RE, Simpson KW. The relationship of mucosal bacteria to duodenal histopathology, cytokine mRNA, and clinical disease activity in cats with inflammatory bowel disease. Vet Microbiol 2008, 128: 178-193.
9. Xenoulis PG, Palculict B, Allenspach K, Steiner JM, Van House AM, Suchodolski JS. Molecular-phylogenetic characterization of microbial communities imbalances in the small intestine of dogs with inflammatory bowel disease. FEMS Microbiol Ecol 2008, 66: 579-589.
10. Packey CD, Sartor RB. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr Opin Infect Dis 2009, 22: 292-301.
11. Craven M, Dogan B, Schukken A, Volkman M, Chandler A, McDonough PL, Simpson KW. Antimicrobial resistance impacts clinical outcome of granulomatous colitis in boxer dogs. J Vet Intern Med 2010, 24: 819-824.
12. Suchodolski, JS, Xenoulis PG, Paddock CG, Steiner JM, Jergens AE. Molecular analysis of the bacterial microbiota in duodenal biopsies from dogs with idiopathic inflammatory bowel disease. Vet Microbiol 2010, 142: 394-400.
13. Suchodolski JS, Dowd SE, Wilke V, Steiner JM, Jergens AE. 16S rRNA Gene Pyrosequencing Reveals Bacterial Dysbiosis in the Duodenum of Dogs with Idiopathic Inflammatory Bowel Disease. Plos One 2012, 7:e39333. doi: 10.1371/journal.pone.0039333.
14. Johnston KL. Small intestinal bacterial overgrowth. Vet Clin North Am Small Anim Pract 1999, 29: 523-550.
15. Johnston KL, Lamport A, Ballevre O, Batt RM. A comparison of endoscopic and surgical collection procedures for the analysis of the bacterial flora in duodenal fluid from cats. Vet J 1999, 157: 85-89.
16. Fragkou FC, Adamama-Moraitou KK, Poutahidis T, Prassinos NN, Kritsepi-Konstantinou M, Xenoulis PG, Steiner JM, Lidbury JA, Suchodolski JS, Rallis TS. Prevalence and Clinicopathological Features of Triaditis in a Prospective Case Series of Symptomatic and Asymptomatic Cats. J Vet Intern Med 2016, 30: 1031-1045.
17. Xenoulis PG, Steiner JM. Canine and feline pancreatic lipase immunoreactivity. Vet Clin Pathol 2012, 41: 312–324.
18. Van den Ingh TS, Cullen JM, Twedt DC, Van Winkle T, Desmet VJ, Rothuizen J. Morphological classification of biliary disorders of the canine and feline liver. In: WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases. Rothuizen J, Bunch SE, Charles JE, Cullen JM, Desmet VJ, Szatmari V, Twedt DC, Van den Ingh TS, Van Winkle T, Washabau RJ (eds). Elsevier: Philadelphia, 2006, pp. 68-71.
19. De Cock HEV, Forman MA, Farver TB, Marks SL. Prevalence and histopathologic characteristics of pancreatitis in cats. Vet Pathol 2007, 44: 39-49.
20. Washabau RJ, Day MJ, Willard MD, Hall EJ, Jergens AE, Mansell J, Minami T, Bilzer TW. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med 2010, 24: 10-26.
21. Quinn PT, Carter ME, Markey BQ, Carter GR. Clinical Veterinary Microbiology. Mosby, 1999.
22. Mehta CR, Patel NR. Exact logistic regression: theory and examples. Stat Med 1995, 14: 2143-2160.
23. Xenoulis PG, Palculict B, Allenspach K, Steiner JM, Van House AM, Suchodolski JS. Molecular-phylogenetic characterization of microbial communities imbalances in the small intestine of dogs with inflammatory bowel disease. FEMS Microbiology Ecology 2008, 66: 579-589.
24. Hirsch VM, Doige CE. Suppurative cholangitis in cats. J Am Vet Med Assoc 1983, 182: 1223-1226.
25. Brain PH, Barrs VR, Martin P, Baral R, White JD, Beatty JA. Feline cholecystitis and acute neutrophilic cholangitis: clinical findings, bacterial isolates and response to treatment in six cases. J Feline Med Surg 2006, 8: 91.
26. Rothuizen J. Liver-Diseases of the biliary system in cats. In: Small Animal Gastroenterology. Steiner JM (ed). Schlütersche: Hannover, 2008, pp. 275-278.
27. Harvey AM, Gruffydd-Jones TJ. Feline Inflammatory Liver Disease In: Textbook of Veterinary Internal Medicine: diseases of the dog and cat. Ettinger SJ and Feldman EC (eds). 7th edn. Saunders, Elsevier: St Louis, 2010, pp. 1643-1709.
28. Wagner KA, Hartmann FA, Trepanier LA. Bacterial culture results from liver, gallbladder or bile in 248 dogs and cats evaluated for hepatobiliary disease: 1998-2003. J Vet Intern Med 2007, 21: 417-424.
29. Savary-Bataille KCM, Bunch SE, Spaulding KA, Jackson MW, Law JM, Stebbins ME. Percutaneous ultrasound guided cholecystocentesis in healthy cats. J Vet Intern Med 2003, 17: 298.
30. Kaufman AC. Infectious causes of feline hepatobiliary disease. Vet Med 1994, 89: 869-873.
31. Day DG. Feline cholangiohepatitis complex. Vet Clin North Am Small Anim Pract 1995, 25: 375-385.
32. Center SA. Diseases of the gall bladder and biliary tree. In: Small Animal Gastroenterology. Guilford WG, Center SA, Strombeck DR, Williams DA, MeyerDJ (eds). 3rd edn. Saunders: Philadelphia, 1996, pp. 860-888.
33. Center SA. Hepatobiliary infections. In: Infectious Diseases of the Dog and Cat. Greene CE (ed). 2nd edn. WB Saunders: Philadelphia, 1998, pp. 615-625.
34. Lapointe JM, Higgins R, Barrette N, Milette S. Enterococcus hirae enteropathy with ascending cholangitis and pancreatitis in a kitten. Vet Pathol 2000, 37: 282-284.
35. Mayhew PD, Holt DE, McLear RC, Washabau RJ. Pathogenesis and outcome of extrahepatic biliary obstruction in cats. J Small Anim Pract 2002, 43: 247-253.
36. Weiss DJ, Armstrong PJ, Gagne J. Inflammatory liver disease. Semin Vet Med Surg (Small Anim) 1997, 12: 22-27.
37. Zawie DA, Garvey MS. Feline hepatic disease. Vet Clin North Am Small Anim Pract 1984, 14: 1201.
38. enter SA, Rowland PH. The cholangitis/cholangiohepatitis complex in the cat. In: Congress Proceedings of ACVIM Forum. San Francisco, 1994, pp. 766-771.
39. Rallis TS. Liver diseases. In: Canine and Feline Gastroenterology. Rallis TS (ed). 2nd edn. University Studio Press: Thessaloniki, 2006, pp. 227-310.
40. Prasse KW, Mahaffey EA, De Novo R, Cornelius L. Chronic lymphocytic cholangitis in three cats. Vet Pathol 1982, 19: 99-108.
41. Day MJ. Immunohistochemical characterization of the lesions of feline progressive lymphocytic cholangitis/cholangiohepatitis. J Comp Pathol 1998, 119: 135-147.
42. Warren Α, Center S, McDonough S, Chiotti R, Goldstein R, Meseck E, Jacobsen M, Rowland P, Simpson K. Histopathologic features, Immunophenotyping, Clonality, and Eubacterial Fluorescence In Situ Hybridization in Cats with Lymphocytic Cholangitis/ Cholangiohepatitis. Vet Pathol 2011, 48: 627-641.
43. Boomkens SY, Kusters JG, Hoffmann G, Pot RG, Spee B, Penning LC, Egberiink HF, van den Ingh TS, Rothuizen J. Detection of Helicobacter pylori in bile of cats. FEMS Immunol Med Microbiol 2004, 42: 307-311.
44. Greiter-Wilke A, Scanziani E, Soldati S, McDonough SP, Mc- Donough PL, Center SA, Rishniw M, Simpson KW. Association of Helicobacter with cholangiohepatitis in cats. J Vet Intern Med 2006, 20: 822-827.
45. Simpson KW. Is there a direct link between IBD, Cholangitis, and Pancreatitis in cats? (abstract). In: Congress Proceedings of ECVIM-CA. Maastricht, The Netherlands, 2012, pp. 169-171.
46. Kordick DL, Brown TT, Shin K, Breitschwerdt EB. Clinical and pathologic evaluation of chronic Bartonella henselae or Bartonella clarridgeiae infection in cats. J Clin Microbiol 1999, 37: 1536-1547.
47. Simpson KW. Pancreatitis and triaditis in cats: causes and treatment. J Small Anim Pract 2015, 56: 40-49.










 PDF Download
PDF Download Military Veterinary Medicine is inextricably linked with the history of the modern veterinary science. In 1774, just 13 years after the establishment of the First School of Veterinary Medicine in Lyon, the first veterinarians were appointed in the French Army. In Greece, the first veterinarian, the Bavarian Georg Horsch, emerged in 1833 and within the next years (1844), the first Greek Veterinary Officers became widely known, such as Emmanouel Pyllas and Nikolaos Kordikas, with the latter writing in 1853 the first Greek book under the title “Equidae Treatment”. In 1875, Georgios Pilavios was employed by the Greek Army. He is considered as the founder of the Military Veterinary Service.
Military Veterinary Medicine is inextricably linked with the history of the modern veterinary science. In 1774, just 13 years after the establishment of the First School of Veterinary Medicine in Lyon, the first veterinarians were appointed in the French Army. In Greece, the first veterinarian, the Bavarian Georg Horsch, emerged in 1833 and within the next years (1844), the first Greek Veterinary Officers became widely known, such as Emmanouel Pyllas and Nikolaos Kordikas, with the latter writing in 1853 the first Greek book under the title “Equidae Treatment”. In 1875, Georgios Pilavios was employed by the Greek Army. He is considered as the founder of the Military Veterinary Service.




 ΜSSDO: Minimum Statistically Significant Difference Observed at a significance level of P=0,05
ΜSSDO: Minimum Statistically Significant Difference Observed at a significance level of P=0,05
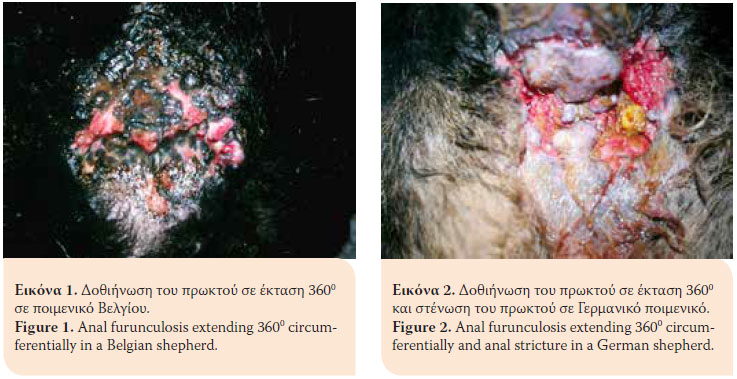
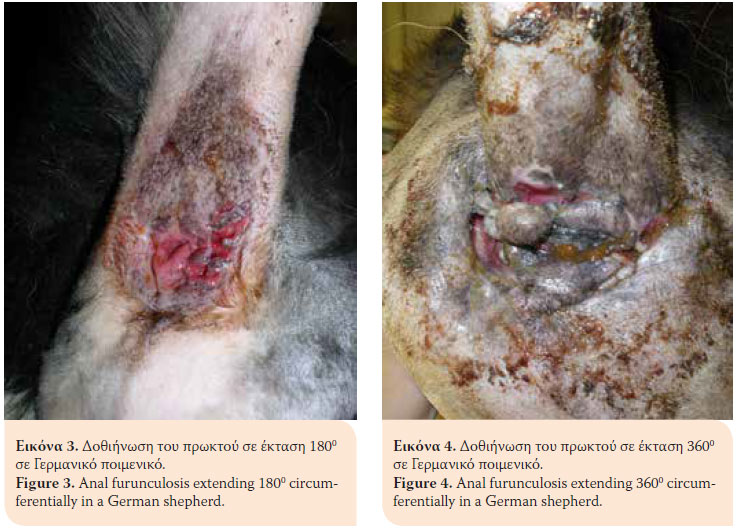
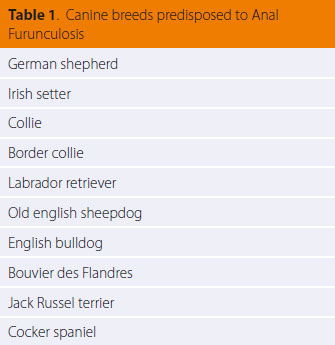 > Introduction
> Introduction
 >Clinical signs
>Clinical signs
 During physical examination, moderately poor body condition was noted (BCS 2/5), with 7% dehydration and abdominal distension during abdominal palpation. The rest of the physical examination was normal. Based on the CIBDAI scale, enteropathy in this case was classified as severe (CIBDAI: 9). CBC was normal, whereas serum biochemistry showed increased ALP activity (307 U/L, reference range: 32-149 U/L), and hypoalbuminaemia (1.8 gr/dL, reference range: 2.9-4 gr/dL). Urinalysis showed reduced urine SG (1026, reference range: >1030) and bilirubinuria (++). Furthermore, levels of serum vitamin Β12 were reduced (180 pg/ ml, reference range: >350 pg/ml). Diagnostic imaging (abdominal radiograph and ultrasound) revealed ascites. Abdominal fluid evaluation classified it as transudate. During gastroscopy (OLYMPUS, XP20) nothing abnormal was observed macroscopically in the oesophagus and stomach. Reaching the small intestine was not possible, due to inability of the endoscope to pass through the pyloric sphincter.
During physical examination, moderately poor body condition was noted (BCS 2/5), with 7% dehydration and abdominal distension during abdominal palpation. The rest of the physical examination was normal. Based on the CIBDAI scale, enteropathy in this case was classified as severe (CIBDAI: 9). CBC was normal, whereas serum biochemistry showed increased ALP activity (307 U/L, reference range: 32-149 U/L), and hypoalbuminaemia (1.8 gr/dL, reference range: 2.9-4 gr/dL). Urinalysis showed reduced urine SG (1026, reference range: >1030) and bilirubinuria (++). Furthermore, levels of serum vitamin Β12 were reduced (180 pg/ ml, reference range: >350 pg/ml). Diagnostic imaging (abdominal radiograph and ultrasound) revealed ascites. Abdominal fluid evaluation classified it as transudate. During gastroscopy (OLYMPUS, XP20) nothing abnormal was observed macroscopically in the oesophagus and stomach. Reaching the small intestine was not possible, due to inability of the endoscope to pass through the pyloric sphincter. Based on history and physical examination findings, chronic PLE and ascites were diagnosed. Prednisolone was administered (1.5 mg/kg BID per os), as well as omeprazole (0.25 mg/ kg SID per os) (LOSEC® 20 mg cap, Astra Zeneca A.E., Αthens, Greece), MCT oil and electrolytes-prebiotics–absorptive substances (Diarsanyl Plus® 10ml/24ml, Ceva, Αthens, Greece), in combination with a clinical gastrointestinal support type diet (Hill’s Prescription Diet Canine i/d®), whereas ascites was managed with furosemide (3 mg/kg BID per os).
Based on history and physical examination findings, chronic PLE and ascites were diagnosed. Prednisolone was administered (1.5 mg/kg BID per os), as well as omeprazole (0.25 mg/ kg SID per os) (LOSEC® 20 mg cap, Astra Zeneca A.E., Αthens, Greece), MCT oil and electrolytes-prebiotics–absorptive substances (Diarsanyl Plus® 10ml/24ml, Ceva, Αthens, Greece), in combination with a clinical gastrointestinal support type diet (Hill’s Prescription Diet Canine i/d®), whereas ascites was managed with furosemide (3 mg/kg BID per os). Aetiologic diagnosis according to histopathology results was lymphoplasmacytic enteritis and secondary lymphangiectasia.
Aetiologic diagnosis according to histopathology results was lymphoplasmacytic enteritis and secondary lymphangiectasia.