Trikoili S. DVM, Companion Animal Clinic, School of Veterinary Medicine, Aristotle University of Thessaloniki | Angelou V. DVM, MSc, PhD, Companion Animal Clinic, School of Veterinary Medicine, Aristotle University of Thessaloniki | Konstantinidis A.O. DVM, MSc, PhD, Companion Animal Clinic, School of Veterinary Medicine, Aristotle University of Thessaloniki | Patsikas M. DVM, MD, PhD, Dip ECVDI, Laboratory of Diagnostic Imaging, School of Veterinary Medicine, Aristotle University of Thessaloniki | Papazoglou L.G. DVM, PhD, MRCVS, Companion Animal Clinic, School of Veterinary Medicine, Aristotle University of Thessaloniki
MeSH keywords: mucocele, biliary sludge, gallbladder rupture, gallbladder, cholecystectomy
Abstract
Gallbladder mucocele (GBM) is now the most common biliary tract disease and the most common indication for cholecystectomy in dogs. GBM is characterized by a complex pathogenesis and involves the accumulation of thick gelatinous bile in the gallbladder. Hyperadrenocorticism, hypothyroidism, hyperlipidemia, hyperleptinemia, hypovitaminosis D, and inflammation of the duodenum predispose to the occurrence of GBM. The clinical signs of dogs with GBM are unclear and the diagnosis is made by ultrasonographic examination. Dogs with biliary sludge should be monitored regularly by ultrasonography since biliary sludge can develop into a mucocele.
Cholecystectomy is preferred to conservative treatment of mucocele because of a better prognosis. Rupture of the gallbladder is a serious complication accompanied by a high mortality rate. Cholecystectomy is imperative to be performed promptly since dogs without clinical signs have lower mortality after cholecystectomy and fewer complications than those with clinical signs.
Introduction
Gallbladder mucocele (GBM) is defined as the distension of the gallbladder due to the accumulation of large amounts of thick, gelatinous bile and mucus within its cavity (Pike et al. 2004, Worley et al. 2004, Malek et al. 2013, Allerton et al. 2018, Jaffey et al. 2018, Parkanzky et al. 2019, Jaffey et al. 2019, Putterman et al. 2021, Friesen et al. 2021, Jaffey et al. 2022, Rossanese et al. 2022, Itoh et al. 2022, Galley et al. 2022). GBM is currently the most common biliary tract disease in dogs and the most common indication for cholecystectomy in this animal species (Putterman et al. 2021, Friesen et al. 2021, Jaffey et al. 2022, Rossanese et al. 2022, Galley et al. 2022, Jaffey 2022).
Pathophysiology
The pathophysiology of the disease appears to be multifactorial and has not been fully elucidated. Recently it was found that the gallbladder epithelium undergoes an increased secretory activity resulting in mucin production and the formation of gelatinous, viscous bile (Kesimer et al. 2015). This increased activity of the gallbladder epithelium is thought to be driven by a phenotypic transformation of yet unknown etiology (Kesimer et al. 2015).
GBM is considered a disease of great significance, since the gradual concentration of viscous bile within the gallbladder can cause: 1) obstruction of the common bile duct, 2) ischemic necrosis due to pressure of the wall of the gallbladder or bile duct, resulting in their rupture and choleperitoneum, 3) cholecystitis, and 4) systematic inflammatory response syndrome (Newell et al. 1995, Crews et al. 2009, Malek et al. 2013, Jaffey et al. 2018, Rogers et al. 2020, Itoh et al. 2022).
The interaction of gallbladder hypomotility, cholestasis, and changes in the bile acid synthesis are thought to be involved in the occurrence of GBM (Tsukagoshi et al. 2012, Kakimoto et al. 2017, Jaffey 2022). Gallbladder hypomotility can cause saturation of bile acids and bile cholesterol causing inflammation of the epithelium, and mucin secretion thus perpetuating hypomotility (Jaffey 2022). The involvement of various endocrinopathies such as hyperadrenocorticism and hypothyroidism, hyperlipidemia, as well as hyperleptinemia, hypovitaminosis D, and duodenal inflammation participate in causing gallbladder hypomotility and altering bile acid synthesis or their interaction resulting in GBM (Mesich et al. 2009, Kutsunai et al. 2014, Jaffey et al. 2019, Viljoen et al. 2021, Jaffey 2022). Biliary sludge, consisting of precipitated cholesterol crystals, calcium, glycoproteins, and mucin, appears to play a significant role in the formation of GBM (Sechi et al. 2012, Tsukagoshi et al. 2012, De Monaco et al. 2016, Mizutani et al. 2017, Butler et al. 2022). It is thought that biliary sludge, especially that which is nongravity dependent and occupies > 50% of the gallbladder volume, is a risk factor for GBM (Butler et al. 2022). The presence of biliary sludge has been found to cause hypomotility or even a reduction in gallbladder ejection fraction resulting in cholestasis (Tsukagoshi et al. 2012, Cook et al. 2016).
Diagnosis
Demographic data
The disease affects older dogs of any gender and breed (Jaffey 2022). In particular, dogs of the breeds Shetland Sheepdog, Cocker Spaniel, Bichon Frise, Shih Tzu, Chihuahua, Pomeranian, Jack Russel Terrier, Beagle, Miniature Schnauzer, Border Terrier, Dachshund, etc. are more commonly affected. (Mesich et al. 2009, Allerton et al. 2017, Rogers et al. 2020, Jaffey et al. 2018, Parkanzky et al. 2019, Jaffey et al. 2019, Friesen et al. 2021, Putterman et al. 2021, Itoh et al. 2022, Rosanesse et al. 2022).
Clinical signs
The clinical signs of dogs vary and may be associated with another concomitant biliary tract disease, with the severity of the disease (subclinical-clinical) or maybe nonspecific (Jaffey et al. 2019, Jaffey 2022) [Table 1]. Generally, in most dogs, the symptomatology is acute with a median duration of 4 days and a range of 1-730 days (Jaffey et al.2019).
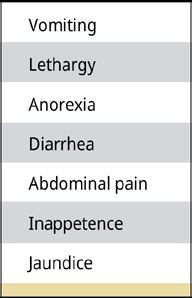
Table 1.
Clinical signs of dogs with clinical GBM with decreasing frequency (Jaffey et al. 2019).
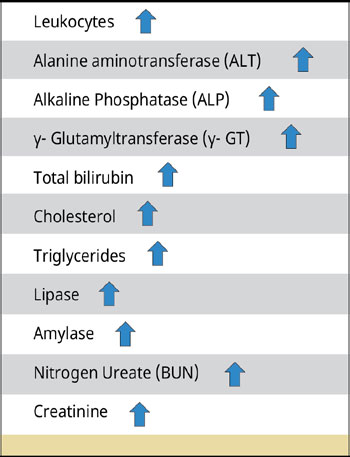
Table 2.
Laboratory picture of dogs with GBM.
Laboratory findings
Laboratory findings vary depending on the severity and chronicity of the condition and the presence of comorbidities (Jaffey 2022). Thus, changes in the general blood test, increased liver enzyme activity, total bilirubin, lipids, urea nitrogen, and creatinine concentrations are observed (Crews et al. 2009, Malek et al. 2013, Choi et al. 2014, Guess et al. 2015, Youn et al. 2018) [Table 2].
Imaging
Ultrasonography is the imaging method of choice for the diagnosis of GBM (Besso et al. 2000, Choi et al. 2013, Jaffey et al. 2022). An ultrasonographic examination can distinguish the presence of GBM and other diseases or conditions of the biliary tract, such as dilatation of the common bile duct, lithiasis of the gallbladder and intra- and extrahepatic bile ducts, and necrosis and/or rupture of the gallbladder. A study in 43 dogs distinguished 6 ultrasonographic types of GBM (Choi et al. 2013) [Table 3] [Figures 1 and 2].
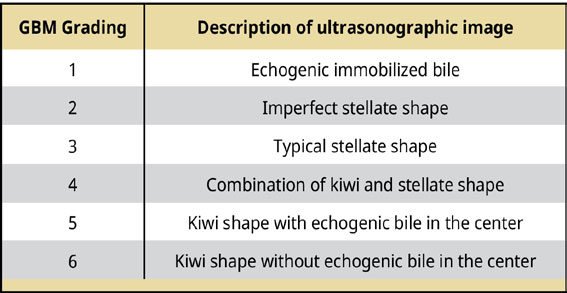
Table 3.
Ultrasonographical grading of GBM (Choi et al. 2013).
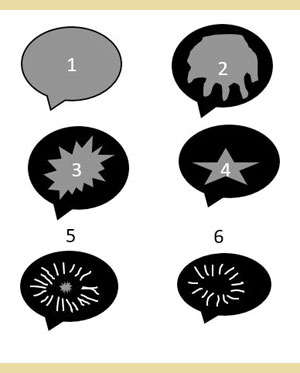
Figure 1. Schematic representation of the 6 types of mucocele according to the ultrasonographic grading by Choi et al. (2014).
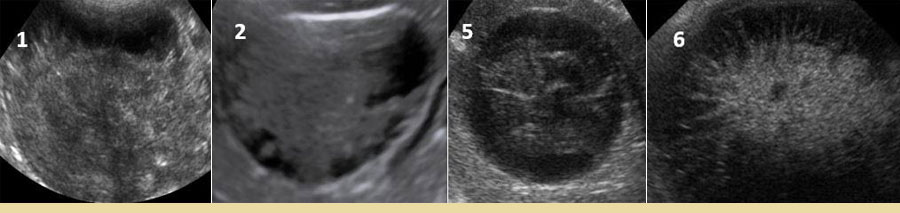
Figure 2.Some types of mucocele (1, 2, 5, and 6) following ultrasonographic examination.
The distinction between GBM and biliary sludge is important, as biliary sludge is found in clinical- ly healthy dogs without biliary pathology (Cook et al. 2016, Butler et al. 2022). In 354 dogs with biliary sludge, it was shown that 6% developed GBM (Cook et al. 2016, Butler et al. 2022). Recently, it was found that dogs with nongravity dependent biliary sludge may develop GBM at some point in their lives and therefore this type of bile is a risk factor for GBM (Butler et al. 2022). It is therefore recommended that ultrasonographic monitoring of dogs with biliary sludge belonging to breeds at high risk of GBM is recommended with scoring of findings as presented in Table 4 (DeMonaco et al. 2016, Cook et al. 2016, Butler et al. 2022). Follow-up could be every 3-6 months, especially in dogs whose owners do not wish to proceed with cholecystectomy (Jaffey 2022).
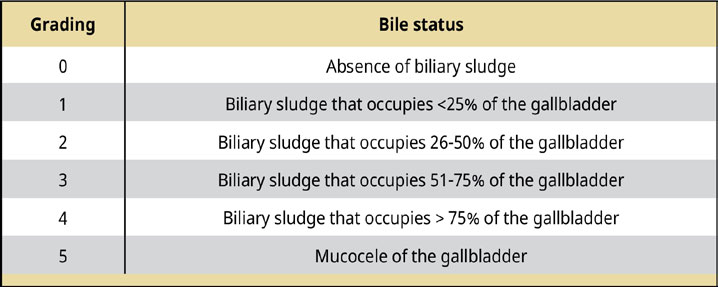
Table 4.
Grading of ultrasonographic findings in dogs with biliary sludge (De Monaco et al. 2016, Cook et al. 2016).
Ultrasonography can also demonstrate the presence of a rupture of the gallbladder wall, an important finding that requires emergency surgical management of the condition (Crews et al. 2009, Jaffey et al. 2018, Wilson et al. 2021). The findings of rupture include discontinuous gallbladder wall with free contents into the peritoneal cavity, hypoechoic fluid collection around the gallbladder, peritonitis in the cranial abdomen, steatitis, and the presence of free contents in the abdominal cavity (Crews et al. 2009, Jaffey et al. 2018, Wilson et al. 2021) [Figure 3]. The sensitivity and specificity of ultrasonographic diagnosis were found to be 56% and 91%, respectively (Jaffey et al. 2018). Suspected gallbladder rupture based on clinical and ultrasonographic findings can be combined with normal total bilirubin concentration (Guess et al. 2015). In one study, 40% of dogs with gallbladder rupture had normal total bilirubin concentration (Wilson et al. 2021). It was also found that when ultrasonography was combined with serum C-reactive protein concentration in blood serum to diagnose gallbladder rupture the sensitivity, specificity, and accuracy of the combination were 100, 93, and 96%, respectively (Asakawa et al. 2022).
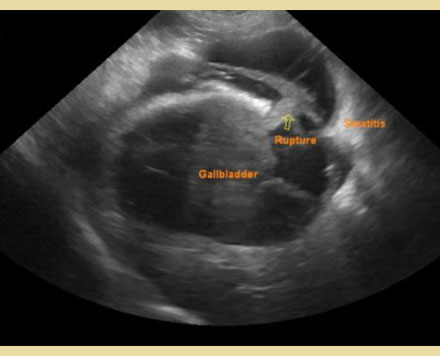
Figure 3. Perforation of mucocele as visualized ultrasonographically (courtesy by Bourdekas P. DVM).
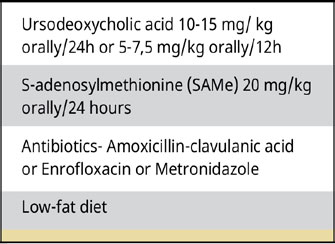
Table 5.
Conservative treatment of dogs with GBM (Parkanzky et al. 2019, Jaffey 2022).
Treatment
The treatment of GBM can be surgical or conservative.
Conservative treatment
Conservative treatment can be applied in dogs with GBM without obstruction in the common bile duct or in the absence of gallbladder rupture, in asymptomatic dogs where GBM was an incidental finding or when surgical treatment is not eligible (Allerton et al. 2018, Parkanzky et al. 2019, Jaffey 2022). Conservative treatment includes choleretics, hepatoprotective agents, and a special diet (Table 5). Antibiotics could be added to the conservative treatment if infection is suspected (Jaffey 2022). A recent study comparing conservative and surgical treatment showed the superiority of surgical treatment for the treatment of GBM. In particular, animals treated surgically had a median survival of 1.802 days, those treated conservatively 1.340 days, while those initially treated conservatively without success followed by surgery had a median survival time of 203 days with the three values differing significantly (Parkanzky et al. 2019).
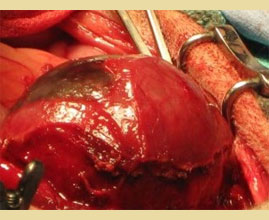
Figure 4. The gallbladder is separated from the hepatic fossa by blunt dissection.
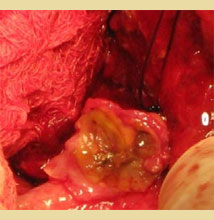
Figure 5. The cystic duct remnant following suture ligation.
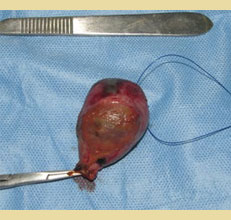
Figure 6. Gallbladder mucocele following cholecystectomy.
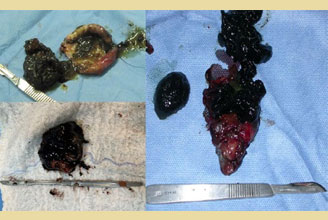
Figure 7. Mucocele was exposed after the opening of the gallbladder in 3 cases.
Surgical treatment
Surgical treatment of GBM is by cholecystectomy and is indicated in dogs with biliary symptomatology, those with evidence or suspicion of common bile duct obstruction, and those with a moderate-acute clinical presentation (Jaffey 2022). Cholecystectomy is performed via midline laparotomy or laparoscopically. Before cholecystectomy by laparotomy, it is mandatory to check the patency of the common bile duct. After dissecting the gallbladder from the hepatic fossa of the liver, which is done either by dissection with a blunt or sharp instrument or finger or in case of adhesions using diathermy up to the bile duct, the gallbladder is excised after ligation of the cystic duct with sutures or vascular clips (Figures 4 -7). Dissection and ligation of the cystic duct must be done carefully because of its not infrequently friable nature. The cystic duct ligation also includes the cystic artery of the gallbladder. In case of bleeding from the hepatic fossa of the liver, its cessation is achieved by tamponade, using hemostatic sponges, or diathermy. (Mayhew & Weisse 2018). During the midline laparotomy, the entire abdominal cavity should be explored to find and remove parts of the mucocele that have escaped from the gallbladder due to rupture or perforation and might have broken into smaller segments (Soppet et al. 2018) [Figures 8, 9 and 10]. Liver biopsies are also recommended since advanced liver fibrosis has been shown to be associated with poor prognosis after cholecystectomy (Jablonski et al. 2023). In case of gallbladder rupture and choleperitoneum after cholecystectomy, the abdominal cavity is lavaged with a large amount of saline followed by the placement of an active drain before closure or by open peritoneal drainage.
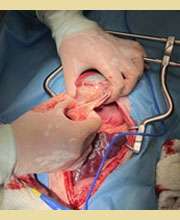
Figure 8. Omental adhesions due to perforation of the gallbladder by a mucocele. Case of Figure 3.
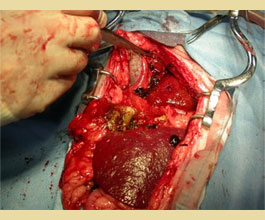
Figure 9. Free mucocele segments disseminated in the abdominal cavity due to gallbladder rupture in a dog.
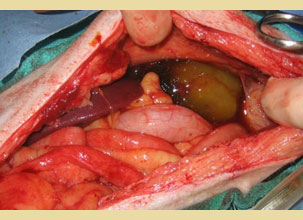
Figure 10. A free mucocele was revealed due to gallbladder rupture through a midline laparotomy in a dog.
Intraoperatively, in case of obstruction of the common bile duct that is diagnosed by ultrasonography (diameter > 4-5 mm) and laboratory tests (high total bilirubin concentration), it is recommended to restore its patency by catheterization of the bile duct either through cholecysteοtomy or retrograde, through catheterization of the major duodenal papilla following enterotomy. Piegols et al. (2021) showed the occurrence of pancreatitis after catheterization and duct flushing regardless of the catheterization method in 252 dogs after cholecystectomy for GBM. Putterman et al. (2021) found that the likelihood of pancreatitis was greater in retrograde common bile duct catheterization compared to that via cholecystotomy in 117 dogs after cholecystectomy for GBM but with a similar survival rate between the two groups. More recently, Rosanese et al. (2022) showed that cholecystectomy for GBM in 82 dogs without common bile duct catheterization was accompanied by a good prognosis. The induction of pancreatitis after retrograde catheterization was probably due either to reflux of secretions into the pancreatic duct during lavage or to injury to the major duodenal papilla by the catheter (Piegols et al. 2021, Putterman et al. 2021). In the opinion of the authors of this review, catheterization of the common bile duct via cholecystotomy should only be performed in case of laboratory, imaging, and intraoperative confirmation of common bile duct obstruction.
As cholestasis predisposes to infection, it is recommended that if infection is suspected, both preoperative and postoperative empiric antibiotherapy that is effective mainly against E. coli and Enterococcus spp. should be given initially until culture and sensitivity test results are available (Jaffey et al. 2018, Galley et al. 2022, Jaffey 2022). Intraoperatively, samples should be taken for culture, both from the bile and from the gallbladder and liver, and preoperative percutaneous gallbladder puncture and material collection should be avoided, as it may cause perforation if the gallbladder wall is friable (Galley et al. 2022, Jaffey 2022). The duration of antibiotherapy arbitrarily ranges from 4 to 6 weeks but no published data are available (Jaffey 2022). It is also recommended that dogs with a negative culture should be given antibiotics postoperatively and avoid perioperative administration that could affect culture results (Jaffey 2022).
Intraoperative and postoperative complications of cholecystectomy
The most common intraoperative and postoperative complications of cholecystectomy for GBM include fever, regurgitations, hypotension, gallbladder rupture or perforation and peritonitis, pancreatitis, sepsis, and death (Worley et al. 2004, Pike et al. 2004, Amsellem et al. 2006, Crews et al. 2009, Piegols et al. 2021, Friesen et al. 2021, Rossanese et al. 2022) [Figure 7]. Gallbladder rupture is a dangerous complication accompanied by a high mortality rate. Dogs with GBM and gallbladder rupture resulting in peritonitis had a 2.7 times higher mortality rate than those with no rupture and peritonitis (Jaffey et al. 2018).
Prognostic indicators of survival after cholecystectomy
The prognosis is generally better for dogs that survive the immediate postoperative period (Malek et al. 2013, Jaffey et al. 2018, Youn et al. 2018, Parkanzky et al. 2019, Piegols et al. 2021, Ullal et al. 2023, Jablonski et al. 2023). Prognostic indicators of survival of dogs undergoing cholecystectomy for GBM are listed in Table 6.
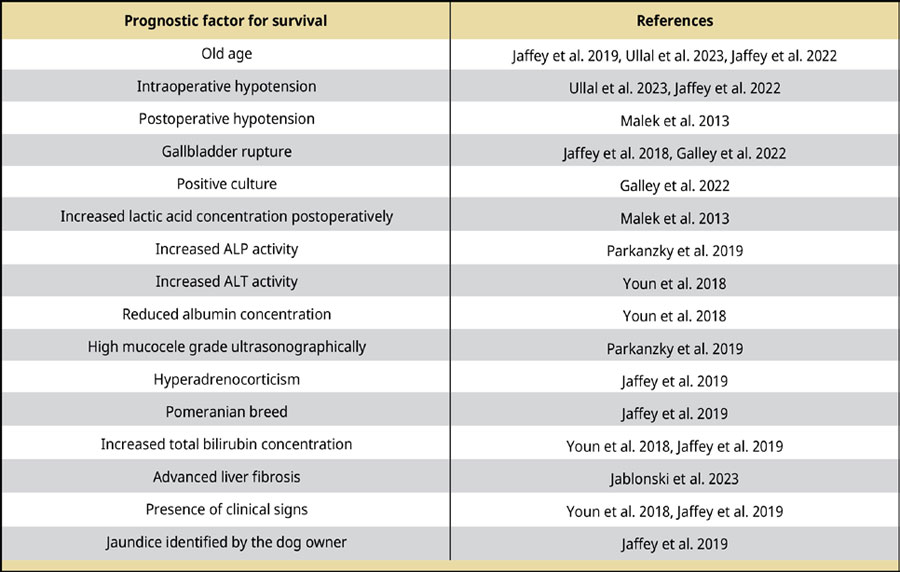
Table 6.
Prognostic factors for survival in dogs after cholecystectomy for GBM.
Time of cholecystectomy for the treatment of GBM
Dogs with GBM that do not have clinical signs have lower mortality ranging from 2-6% and less significant complications than those with clinical signs, with mortality ranging from 17-23% (Youn et al. 2018, Jaffey et al. 2019, Friesen et al. 2021, Jablonski et al. 2023). Based on the above findings, early cholecystectomy is recommended in dogs with mild-subclinical GBM, resulting in a reduced risk of postoperative mortality and no major complications. However, it should be noted that cholecystectomy is not a risk-free procedure since many dogs with subclinical GBM do not survive in the immediate postoperative period (Amsellem et al. 2006, Wallace 2022).
In conclusion, GBM is currently the most common indication for cholecystectomy in dogs. Dogs with GBM should be screened for hypothyroidism and hyperadrenocorticism which are predisposing factors for GBM. Dogs with biliary sludge should be monitored regularly by ultrasonography since biliary sludge can lead to GBM. Catheterization of the common bile duct, when required, should be performed via cholecystotomy because retrograde catheterization is associated with the development of pancreatitis. Gallbladder rupture is a serious complication associated with a high mortality rate. Dogs with GBM without clinical symptoms have lower mortality after cholecystectomy and less significant complications than those with clinical symptoms. Preventive cholecystectomy should be performed in cases of GBM with subclinical or mild symptomatology as the efficacy of conservative treatment has not been elucidated. Early intervention is associated with reduced mortality rates.
Conflict of interest
The authors declare that there is no conflicts of interest.
Corresponding author:
L. G. Papazoglou
This email address is being protected from spambots. You need JavaScript enabled to view it.
References
- Allerton F, Swinbourne F, Barker L, Black V, Kathrani A, Tivers M, Henriques T, Kisielewicz C, Dunning M, Kent A (2018) Gall bladder mucoceles in border terriers. J Vet Intern Med 32, 1618-1628.
- Amsellem PM, Seim III HB, MacPhail CM, Bright RM, Twedt DC, Wrigley RH, Monnet E (2006) Long-term survival and risk factors associated with biliary surgery in dogs: 34 cases (1994-2004). J Am Vet Med Assoc 229, 1451-1457.
- Asakawa M, Fukuzawa M, Asakawa MG, Flanders JA (2022) Preoperative serum C- reactive protein concentration can be used to detect gallbladder rupture in dogs with gallbladder mucocele. Am J Vet Res 83, 23-32.
- Besso JG, Wrigley RH, Gliatto JM, Webster CRL (2000) Ultrasonographic appearance and clinical findings in 14 dogs with gallbladder mucocele. Vet Radiol Ultrasound 41, 261-271.
- Butler T, Bexfield N, Dor C, Fantaconi N, Heinsoo I, Kelly D, Kent A, Pack M, Spence SJ, Ward PM, Watson P, McCallum KE (2022) A multicenter retrospective study assessing progression of biliary sludge in dogs using ultrasonography. J Vet Intern Med 36, 976-985.
- Choi J, Kim A, Oh SKJ, Kim H, Yoon J (2014) Comparison between ultrasonographic and clinical findings in 43 dogs with gallbladder mucoceles. Vet Radiol Ultrasound 55, 202-207.
- Cook AK, Anisha Jambhekar V, Dylewski AM (2016) Gallbladder sludge in dogs: ultrasonographic and clinical findings in 200 patients. J Am Anim Hosp Assoc 52, 125-131.
- Crews LJ, Feeney DA, Jessen CR, Rose ND, Matise I (2009) Clinical, ultrasonographic, and laboratory findings associated with gallbladder disease and rupture in dogs: 45 cases (1997-2007). J Am Vet Med Assoc 234, 359-366.
- DeMonaco SM, Grant DC, Larson MM, Panciera DL, Leib MS (2016) Spontaneous course of biliary sludge over 12 months in dogs with ultrasonographically identified biliary sludge. J Vet Intern Med 30, 771-778.
- Friesen SL, D. Upchurch A, Hollenbeck DL, Roush JK (2021) Clinical findings for dogs undergoing elective and nonelective cholecystectomies for gall bladder mucoceles. J Small Anim Pract 62, 547-563.
- Galley M, Lang J, Mitchell M, Fletcher J (2022) Factors affecting survival in 516 dogs that underwent cholecystectomy for the treatment of gallbladder mucocele. Can Vet J 63, 63-66.
- Guess SC, Harkin KR, Biller DS (2015) Anicteric gallbladder rupture in dogs: 5 cases (2007-2013). J Am Vet Med Assoc 247, 1412-1414.
- Itoh H, Igari K, Tani K, Sunahara H, Nemoto Y, Nakaichi M, Iseri T, Horikirizono H, Itamoto K (2022) Clinical relationship between histopathological necrotic/partial necrotic findings and disease condition of gallbladder mucoceles in dogs. Polish J Vet Sci 25, 223-229.
- Jablonski SA, Chen YXP, Williams JE, Kendziorski JA, Smedley RC (2023) Concurrent hepatopathy in dogs with gallbladder mucocele: prevalence, predictors and impact on long-term outcome. J Vet Intern Med, https://doi.org/10.1111/jvim.16922.
- Jaffey JA, Graham A, VanEerde E, Hostnik E, Alvarez W, Arango J, Jacobs C, Declue AE (2018) Gallbladder mucocele: variables associated with outcome and the utility of ultrasonography to identify gallbladder rupture in 219 dogs (2007-2016). J Vet Intern Med 32, 195-200.
- Jaffey JA (2022) Canine extrahepatic biliary disease. J Small Anim Pract 63, 247-264.
- Jaffey JA, Kreisler R, Shumway K, Jane-Lee Y, Hui-Lin C, Durocher-Babek LL, Won-Seo K, Choi H, Nakashima K, Harada H, Kanemoto H, Shuan-Lin L (2022) Ultrasonographic patterns, clinical findings, and prognostic variables in dogs from Asia with gallbladder mucocele. J Vet Intern Med 36, 565-575.
- Jaffey JA, Pavlick M, Webster CR, Moore GE, McDaniel KA, Blois SL, Brand EM, Reich CF, Motschenbacher L, Hostnik ET, Su D, Lidbury JA, Raab O, Carr SV, Mabry KE, Fox-Alvarez W, Townsend S, Palermo S, Nakazono Y, Ohno K, VanEerde E, Fieten H, Hulsman AH, Cooley-Lock K, Dunning M, Kisielewicz C, Zoia A, Caldin M, Conti-Patara A, Ross L, Mansfield C, Lynn O, Claus MA, Watson PJ, Swallow A, Yool DA, Gommeren K, Knops M, Ceplecha V, De Rooster H, Lobetti R, Dossin O, Jolivet F, Papazoglou LG, Pappalardo MCF, Manczur F, Dudás-Györki Z, O’Neill EJ, Martinez C, Gal A, Owen RL, Gunn E, Brown K, Harder LK, Griebsch C, Anfinsen KP, Gron TK, Marchetti V, Heilmann RM, Pazzi P, DeClue AE (2019) Effect of clinical signs, endocrinopathies, timing of surgery, hyperlipidemia, and hyperbilirubinemia on outcome in dogs with gallbladder mucocele. Vet J 251, 105350.
- Kakimoto T, Kanemoto H, Fukushima K, Ohno K, Tsujimoto H (2017) Bile acid composition of gallbladder contents in dogs with gallbladder mucocele and biliary sludge. Am J Vet Res 78, 223229.
- Kesimer M, Cullen J, Cao R, Radicioni G, Mathews KG, Seiler G, Gookin JL (2015) Excess secretion of gel-forming mucins and associated innate defense proteins with defective mucin un-packaging underpin gallbladder mucocele formation in dogs. PLoS ONE 10, e0138988.
- Kutsunai M, Kanemoto H, Fukushima K, Fujino Y, Ohno K, Tsujimoto H (2014) The association between gall bladder mucoceles and hyperlipidaemia in dogs: a retrospective case control study. Vet J 199, 76-79.
- Malek S, Sinclair E, Hosgood G, Moens NMM, Baily T, Sarah E. Boston SE (2013) Clinical findings and prognostic factors for dogs undergoing cholecystectomy for gall bladder mucocele. Vet Surg 42, 418-426.
- Mayhew PD, Weisse C (2018) Liver and Biliary System. In: Veterinary Surgery: Small Animal. Johnston SA, Tobias KM eds. Elsevier, St Louis, pp 1829-1852.
- Mesich MLL, Mayhew PD, Paek M, Holt DE, Brown DC (2009) Gallbladder mucoceles and their association with endocrinopathies in dogs: a retrospective case-control study. J Small Anim Pract 50, 630-635.
- Mizutani S, Torisut S, Kaneko Y, Yamamoto S, Fujomoto S, Ong BHE, Nacanobu K (2017) Retrospective analysis of canine gallbladder contents in biliary sludge and gallbladder mucoceles. J Vet Med Sci 79, 366-374.
- Newell SM, Selcer BA, Mahaffey MB, Gray ML, Jameson PH, Cornelius LM, Downs MO (1995) Gallbladder mucocele causing biliary obstruction in two dogs: ultrasonographic, scintigraphic, and pathological findings. J Am Anim Hosp Assoc 31, 467-472.
- Parkanzky M, Grimes J, Schmiedt C, Secrest S, Bugbee A (2019) Long-term survival of dogs treated for gallbladder mucocele by cholecystectomy, medical management, or both. J Vet Intern Med 33, 2057-2066.
- Piegols HJ, Hayes GM, Lin S, Singh A, Langlois DK, Duffy DJ (2021) Association between biliary tree manipulation and outcome in dogs undergoing cholecystectomy for gallbladder mucocele: a multi-institutional retrospective study. Vet Surg 50, 767-774.
- Pike FS, Berg J, King NW, Penninck DG, Webster CRL (2004) Gallbladder mucocele in dogs: 30 cases (20002002). J Am Vet Med Assoc 224, 1615-1622.
- Putterman AB, Selmic LE, Kindra C, Duffy DJ, Risselada M, Phillips H (2021) Influence of normograde versus retrograde catheterization of bile ducts in dogs treated for gallbladder mucocele. Vet Surg 50, 784-793.
- Rogers E, Jaffey JA, Graham A, Hostnik ET, Jacobs C, Fox-Alvarez W, Van Eerde E, Arango J, Williams F, DeClue AE (2020) Prevalence and impact of cholecystitis on outcome in dogs with gallbladder mucocele. J Vet Emerg Crit Care 30, 97-101.
- Rossanese M, Williams P, Tomlinson A, Cinti F (2022) Long-term outcome after cholecystectomy without common bile duct catheterization and flushing in dogs. Animals 12, 2112.
- Secchi P, Poppl AG, Ilha A, Kunert Filho HC, Lima FES, Garcia AB, Conzalez FHD (2012) Prevalence, risk factors, and biochemical markers dogs with ultrasound-diagnosed biliary sludge. Res Vet Sci 93, 1185-1189.
- Soppet J, Young BD, John F. Griffin JF IV, Gilmour LJ, Heffelman V, Tucker-Mohl K, Biller DS, Wolff, CA Spaulding KA (2018) Extruded gallbladder mucoceles have characteristic ultrasonographic features and extensive migratory capacity in dogs. Vet Radiol Ultrasound 59, 744-748.
- Tsukagoshi T, Ohno K, Tsukamoto A, Fukushima K, Takahashi M, Nakashima K, Fujino Y, Tsuijimoto H (2012) Decreased gallbladder emptying in dogs with biliary sludge or mucocele. Vet Radiol Ultrasound 53, 84-91.
- Ullal TV, Jaffey JA, Kreisler R, Matheson J, Pacholec C, Shumway K, Van der Bossche L, Fieten H, Ringold R, DeClue AE (2023) Increasing age and severe intraoperative hypotension associated with nonsurvival in dogs with gallbladder mucocele undergoing cholecystectomy. J Am Vet Med Assoc doi: 10.2460/javma.23.06.0305.
- Viljoen AD, Tamborini A, Watson PJ, Bexfield NH (2021) Clinical characteristics and histology of cholecystectomised dogs with nongravity-dependent biliary sludge:16 cases (2014-2019). J Small Anim Pract 62, 478-488.
- Wallace ML (2022) Updates in hepatobiliary surgery. Vet Clin Small Anim Pract 52, 369-385.
- Wilson K, Powers D, Grasperge B, Liu CC, Granger LA (2021) Dogs with biliary rupture based on ultrasound findings may have normal total serum bilirubin values. Vet Radiol Ultrasound 62, 236-245.
- Worley DR, Hottinger HA, Lawrence HJ (2004) Surgical management of gallbladder mucoceles in dogs: 22 cases (1999-2003). J Am Vet Med Assoc 225, 1418-1422.
- Youn G, Michelle Waschak J, Kunkel KAR, Gerard PD (2018) Outcome of elective cholecystectomy for the treatment of gallbladder disease in dogs. J Am Vet Med Assoc 252, 970-975.


