> Abstract
Corneal damage in dog and cat can alter its clarity and transparency in light, which are essential for its function. Corneal lesions include edema, neovascularisation, pigmentation, microcrystal depositions, ulceration, inflammation or regenerative tissue formation, scar formation and finally those involving its size and curvature. This lesion may occur solely or in conjunction and may be caused by corneal, other ophthalmic or systemic diseases. In this study the above mentioned corneal lesions of the dog and cat are extensively reported and described.
> Introduction
Cornea is the frontier, clear and transparent in light part of the fibrous tunic of the eye. Cornea is the first and most important diopter of the eye’s refractive structures, since it ensures the 2/3 convergence of light rays on the retina. Any corneal damage, which leads to change of its size, shape or transparency, may alter more or less the vision. Clinicians should to be able to understand the pathophysiology of corneal lesions, as well as to identify and describe them, in order to be able to correlate them with certain diseases. This study describes the most commonly presented corneal lesions of the dog and cat in everyday practice.
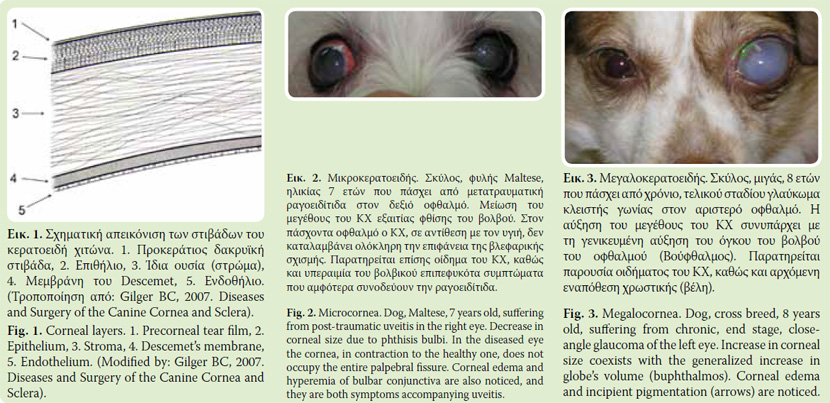
Anatomy and physiology1, 2, 3
Mean corneal thickness in the dog is 0,562mm4 and in the cat5 0,546mm and it consists from outwards to inwards of:
- Anterior epithelium
- Stroma
- Descemet’s membrane
- Endothelium
In these four anatomical layers of cornea one more has to be added (Fig. 1), the precorneal tear film (PTF).6
Corneal integrity is essential for vision quality, while its role is mechanical and optical.
Mechanically, cornea contributes in the formation of eye globe, supporting and protecting its inner anatomical structures. It is worth to be mentioned, in regards to its fine structure, the high resistance of cornea to elevated intraocular pressure and environmental injuries.
Optically, cornea functions as a convergent lens. Moreover, it is the most important convergent lens of the eye’s refractive system, as its convergence capability is 42 D. Corneal function is correlated to its own clarity and transparency in light. These are based on:
1. Normal corneal curvature and smooth optical surface. Normal curvature depends on the anatomical integrity of cornea. Its smooth and shiny surface is provided by the PTF.
2. Nonkeratinized surface epithelium. Corneal epithelium is stratified squamous epithelium with high regenerative capability. It consists of 7-9 cell layers. The anterior layers are not keratinized in order to remain transparent.
3. Lack of blood vessels. Healthy cornea is avascular. Nutrition is provided in a limited level by the limbus vessels and mainly from PTF through epithelium and from aqueous humor through endothelium. In contrast to vascularisation, sensitivity of cornea is very extended and is considered as one of body’s most sensible tissues. Nerve endings are provided from ciliary nerves, which are the ending branches of the ophthalmic branch of trigeminal nerve. Nerve endings are distributed into epithelium and superficial stromal layer which may explain that superficial corneal lesions are more painful than deeper ones.
4. Special stromal architecture, which forms 90% of total cornea’s thickness. Stroma is mainly consisted of lamellae of fibrous tissue, few fibrocytes (keratocytes) and interstitial matrix (glycosaminoglycans). The architectural arrangement of stromal collagen fibrils and lamellae differs from those of other connective tissue, providing stromal transparency.
5. Regulation of stromal humidity level. Stromal transparency depends on the maintenance of stromal humidity level. Increase in corneal humidity leads to corneal edema and loss of transparency. The integrity of both epithelium and endothelium is essential for maintaining the hydrostatic state of stroma. Epithelium is water resistant and consisting a barrier to water diffusion from PTF to stroma. Endothelium, even though consisting of a single cell layer, is responsible for energy-dependent ion and water transfer from stroma to aqueous humor and anterior chamber.
> CORNEAL LESIONS
1. Size and curvature abnormalities
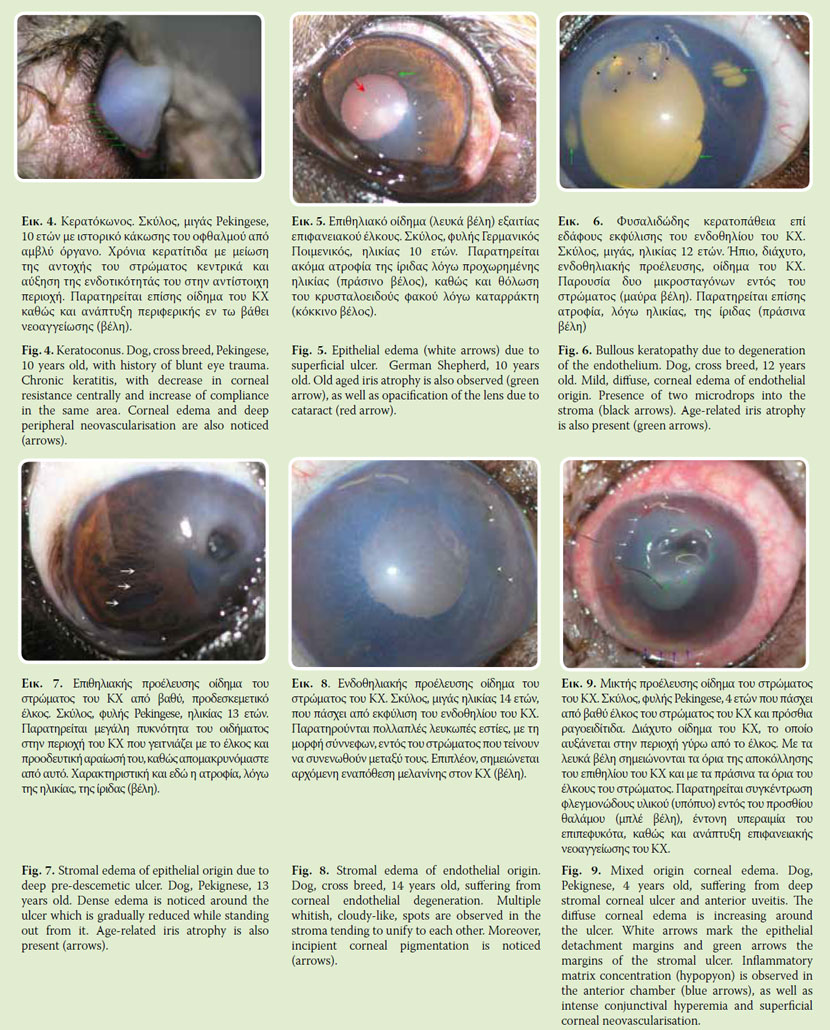
Microcornea: The decrease of corneal size may be congenital, as a part of microphthalmia7 or acquired following a phthisis bulbi (Fig. 2).
Magalocornea: Magalocornea is a rare condition, usually congenital.7 When acquired it follows the total increase in bulbi size, due to increased intraocular pressure (end stage chronic glaucoma- buphthalmus) (Fig. 3).
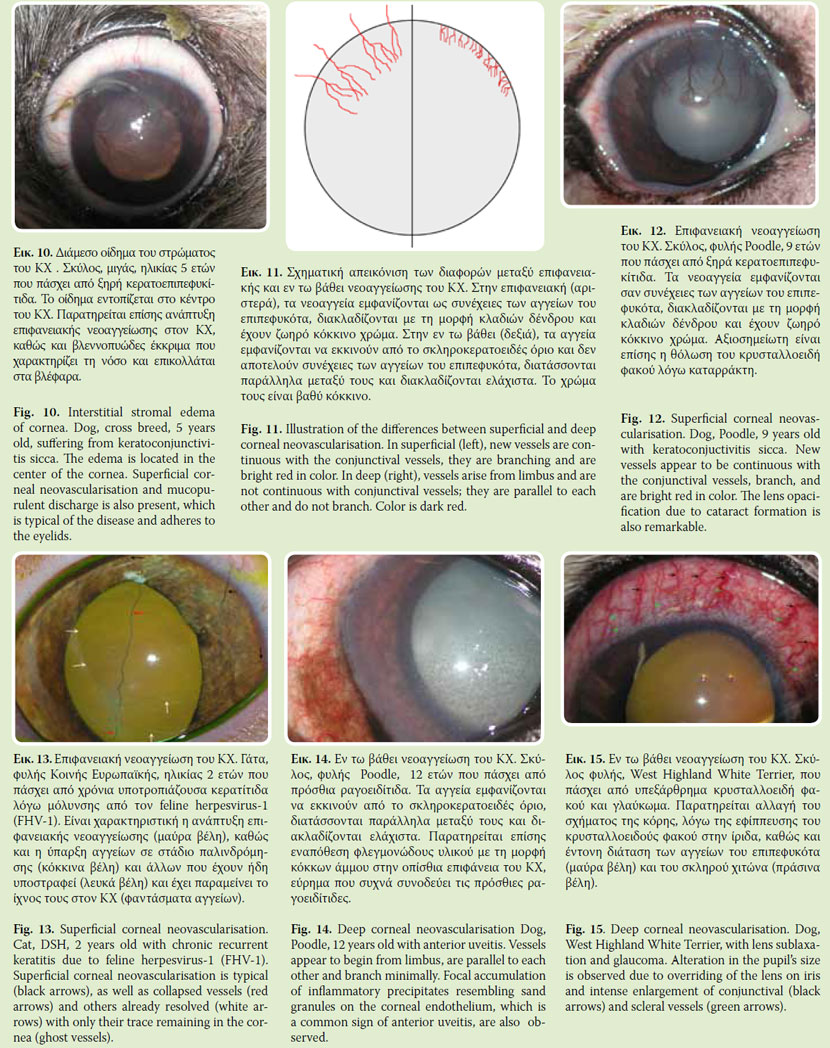
Ceratoconus. Unlike human, in dogs and cats ceratoconus is not a primary disease. It is usually secondary to chronic keratitis or central corneal ulcer, which results in central corneal thinning and increased compliance centrally (Fig. 4).
2. Corneal edema7
Edema is the most common corneal defect. Usually it coexists with other corneal defects. Corneal edema is characterized by the overhydration of corneal tissue, which leads to corneal opacity and decreased transparency. There are several causes of corneal edema and sometimes more than one can occur concurrently. Corneal edema can be focal or diffuse and concerns epithelium, stroma or both.
Epithelial edema (Fig. 5) usually is mild. In slit lamp biomicroscopy epithelial edema appears like a vapour on a pane of glass. Following installation of glycerine dilute, epithelial edema is temporally disappeared. Epithelial edema is caused by erosion or loss of the epithelium, which results in imbibition of PTF fluid by the inner epithelium layers or superficial stroma. For this reason the edema is confined to the area of epithelial defect.
Stromal edema appears as a mild or severe corneal opacity which in some cases may appear milky. In the beginning, slit lamp biomicroscopy reveals multiple white corneal spots with haze edges which tend to join each other. Upon slit lamp examination low or high degree of corneal thickness (proportional to the degree of edema) is typically observed. A special form of stromal edema is bullous keratopathy, in which there is not a corneal fluid imbibition by stromal interstitial tissue but micro-drops accumulation in the stroma7 (Fig. 6). Causes of corneal edema can be epithelial, endothelial or both in origin. A unique form of edema is the interstitial stromal edema in which epithelium, as well as endothelium is intact.
Stromal edema of epithelial origin is caused by the disruption of the epithelial barrier between PTF and stroma, due to epithelial injury (Fig. 7). This results in absorbing water by glycoaminoglycanes of stromal interstitial substance. Corneal ulceration is the most common cause of epithelial origin edema. In these cases stromal edema is located in and around the lesion and its density is proportional to its extension, depth and chronicity.
Stromal edema of endothelial origin (Fig. 8) is caused by endothelial functional incapability to move water from the stroma to aqueous chamber. 3-7 In these cases the flow is reversed and aqueous humor enters stroma. Decrease in endothelial functional capacity is commonly caused by inflammation (endothelitis), which may be primary or secondary to anterior uveitis.8 Typical stromal edema of endothelial origin is noticed in canine adenovirus- 1 infection (CAV-1).9 Less common causes of endothelial damage and subsequent stromal edema formation is endothelial dystrophy and degeneration, 10-11 iris anterior synechiae, as well as trauma during intraocular procedures.12 The extension and density of edema is also related to the severity of endothelial injury.
Corneal edema of mixed, epithelial and endothelial, origin is caused by injury of both epithelium and endothelium (Fig. 9). In these cases endothelial injury causes a diffuse homogenous corneal edema, while epithelial regional defect increases locally its density.
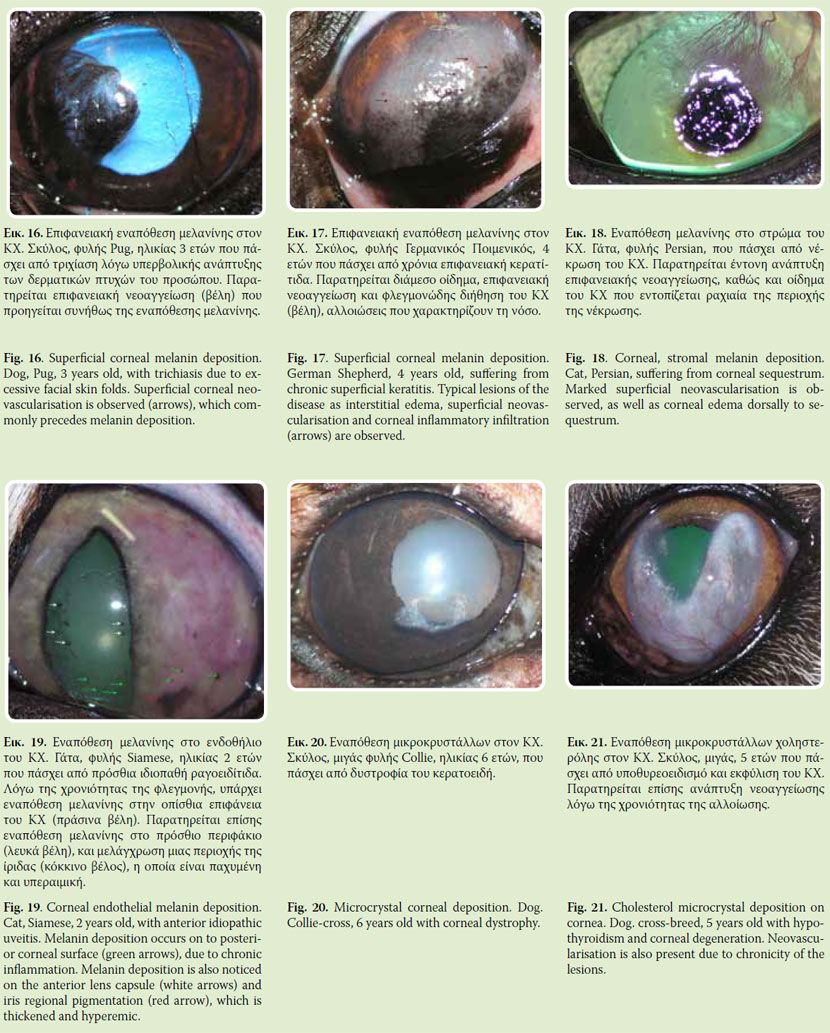
In interstitial stromal edema both epithelium and endothelium are intact (Fig. 10). Initially, it is peripheral and is extended gradually centrally to the cornea. Interstitial keratitis is the most common cause of interstitial edema which is commonly accompanied by inflammatory cell infiltration. Another form of stromal interstitial edema is the one accompanying canine leishmaniosis, even though in these cases edema is caused mainly by anterior uveitis and endothelitis.
3. Corneal Neovascularisation13
Normal cornea is avascular. Vascularisation beyond limbus, as part of corneal tissue defense function is always pathologic and leads to decrease corneal transparency. The form, density and depth of vascularisation is of high diagnostic importance. Morphologically, corneal new-vessels may form bunches, branches or may expand circularly to limbus.
Density of neovasularisation may vary and is correlated to the severity of the underlying disease. The density and the length of corneal new-vessels are proportional to chronicity of the lesion, as their development does not exceed 1-2mm/24h.
Corneal neovascularisation may be either superficial or deep (Fig. 11). In superficial vascularisation (Fig. 9, 10, 11), new-vessels are derived from limbus vessels and are continuous with the conjunctival vessels. They are located in the corneal epithelial layer or underneath; they form brightly red colored branches. Superficial neovascularisation is related to acute or chronic superficial corneal lesions (e.g. superficial ulcers, superficial keratitis). Frequently, only vascular walls (ghost vessels) are observed in cornea, which indicate previous corneal vascularisation (Fig. 13). In deep corneal neovascularisation (Fig. 14, 15) newvessels begin from ciliary vessels; and thus they appear to start from limbus and they are not continuous with the conjunctival vessels. They are located in stroma, in parallel order and are minimally branched. Frequently they have a brush appearance. They are dark red. Deep corneal neovascularisation is related to stromal diseases (e.g. deep ulcers, interstitial keratitis), as well as to uveitis and glaucoma.
4. Corneal pigmentation
Corneal pigmentation is most commonly presented in the dog than in the cat. Melanin is the most common pigment, while infrequently pigmentation with hemoglobin or metal dyes due to foreign metal body fixation in the cornea, may occur.
4α. Melanin deposition13
Melanin is the most common pigment deposited on the cornea. It suggests chronic lesion. Cornea melanin originates from limbal, ciliary body and iris melonocytes. Transportation takes place through vessels, thus it is highly correlated with neovascularisation. Melanin, depending on its cause, is accumulated superficially and more rarely in stroma or endothelium. Epithelial deposition of melanin suggests complication of chronic corneal edema or chronic corneal irritation (e.g. distichiasis, trichiasis, entropion, corneal overexposure due to exophthalmos etc.) (Fig. 16). Moreover, superficial accumulation of melanin accompanies frequently or follows superficial inflammations, such as chronic superficial keratitis and keratoconjunctivitis sicca (Fig. 17). Stromal deposition of melanin is observed mainly in corneal sequestrum of the cat14 (Fig. 18). Deep deposition of melanin is less common and usually it is noticed in chronic anterior uveitis (Fig. 19). In such cases, melanin granules, originated from the inflamed anterior uvea, swing in the aqueous humor and accumulate on the posterior corneal surface.
4β.Hemoglobin deposition
Corneal hemoglobin deposition is rare. It results from anterior chamber hemorrhage and stromal infiltration of hemoglobin through endothelium and Descemet’s membrane. Hemoglobin deposition in the beginning is brownish in color
5. Microcrystal deposition7
It concerns lipid, cholosterole or calcium microcrystal deposits of metallic lustre in the cornea. They are usually bilateral and symmetrical. Usually they are correlated with corneal dystrophy or degeneration (Fig. 20). Rarely are they associated with systemic diseases, which cause alterations in lipids or calcium metabolism (hypothyroidism, diabetes mellitus, pancreatitis) (Fig. 21).
6. Corneal ulceration7, 13, 15
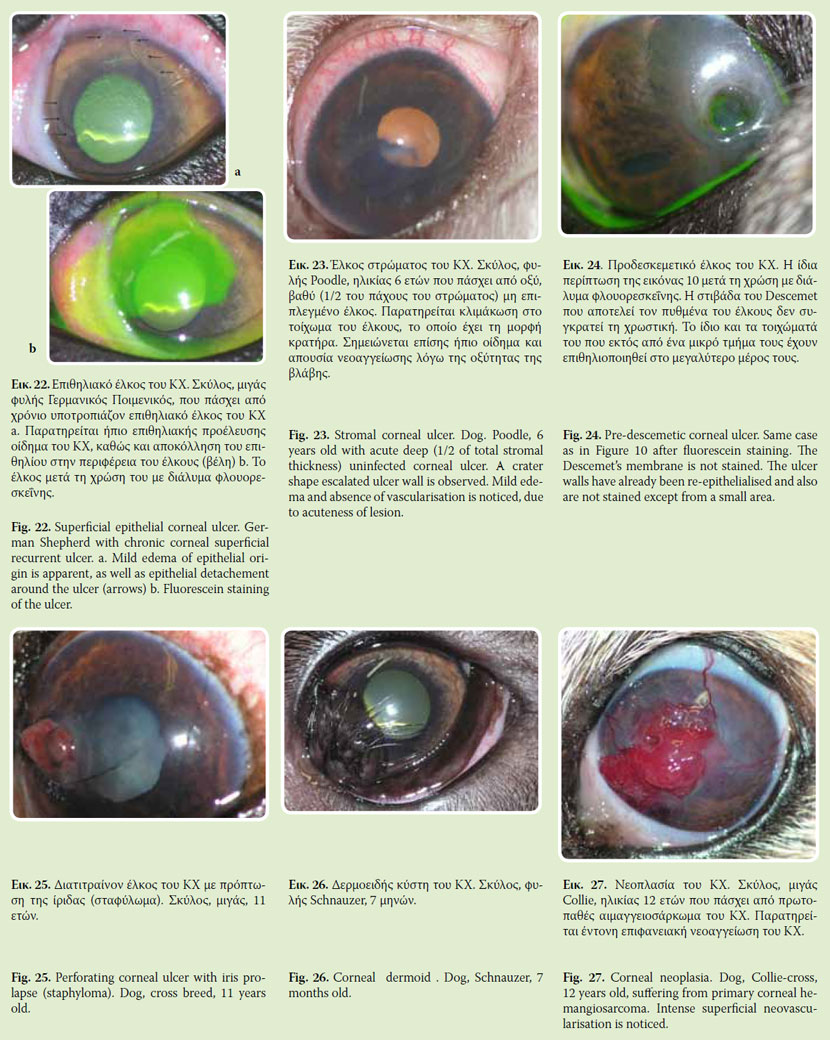
Corneal ulceration refers to any corneal tissue loss. Epithelial ulcers concern only epithelial loss. A particular epithelial ulcer form is the chronic recurrent corneal ulcer (Fig. 22). When it concerns stromal defect, it is characterized as stromal ulcer (Fig. 23). In case of full thickness stromal defect, it is characterized as pre-descemetic ulcer (Fig. 7). In these cases Descemet’s membrane is protruding within the ulcer in various degrees due to its elasticity. It must be mentioned that in most cases, stromal ulcer is accompanied by other lesions (edema, neovasularisation). Fluorescein staining is positive in ulcers although in case of pre-descemetic ulcer, only its vertical walls are stained, if they are not epithelialized and not its floor (Fig. 24). Finally, in Descemet’s membrane rupture the ulcer is characterized as perforated, while from its floor it may protrude a part of iris (staphyloma), which is usually covered by fibrin (Fig. 25).
7. Neoplastic tissue development/ inflammatory reaction
Dermoid is congenital and it results from the development of skin tissue on cornea (Fig. 26). This tissue may include all anatomical features of the skin, as well as hair follicles.16
Several types of corneal neoplasms have been described such as squamous cell carcinoma, papilloma, lymphosarcoma, hemangiomas, hemangiosarcoma and adenocarcinoma.17 They are presented like raised areas, rich in vascularisation (Fig. 27).
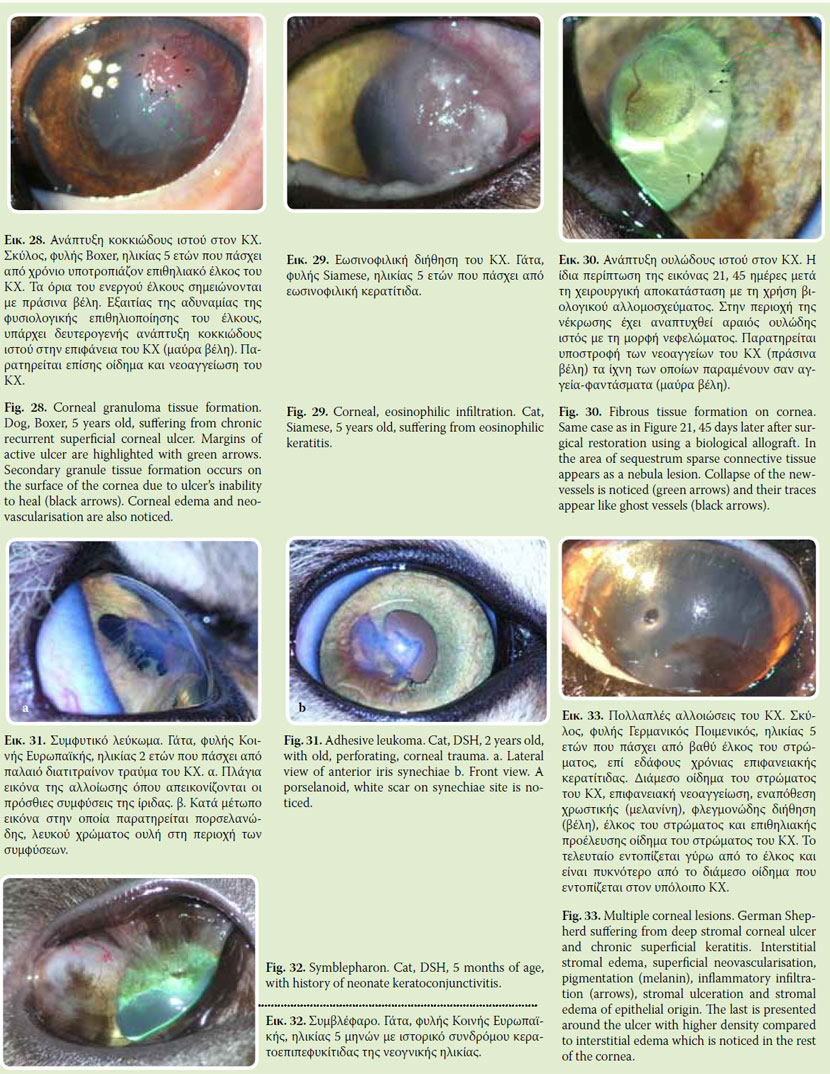
In some chronic keratitis or during ulcer healing process, cornea is infiltrated by inflammatory cells/ granular tissue. This inflammatory infiltrations form smaller or larger red-white raised corneal spots. Usually, they are rich in vascularisation with an irregular surface (Fig. 28). When these infiltrations are located away from limbus they are irrigated from one or two vessels. Occasionally, in the inflamed area erosions are apparent and fluorescein staining is positive but this does not correspond to corneal ulcer. A special form of inflammatory corneal infiltration is the eosinophilic keratitis of the cat in which eosinophils predominate18, 19 (Fig. 29).
8. Scar formation13
Superficial lesions of the cornea concerning epithelium as well as superficial stromal layers heal without scar formation, even though a minimal alteration in corneal curvature may be noticed leading to astigmatism. In deeper stromal lesions scar tissue formation is usual. This is caused by the inability of proper reparation of corneal architecture, which is responsible for its clarity. The scar tissue may have the form of nebula (Fig. 30) which is transparent or of a dense white to percenaloid area which is opaque. A special category of scar formation is the adherent leukoma in which iris attaches cornea posteriorly and suggests previous corneal penetration (Fig. 31). Finally, severe keratoconjunctivities may result in adhesions between cornea and conjunctiva or/and eyelids (symblepharon). These lesions vary in extension and appear frequently in cats suffering from keratoconjunctivitis due to the feline upper respiratory syndrome (Fig. 32).
The aforementioned lesions may appear solitary or in combination (Fig. 33). When more than one lesion coexists, clinician should observe and describe them using a bright light source and appropriate magnifying equipment. Then, history and other findings of the ophthalmic examination are taken under consideration paying attention in the sequence in which these signs first appeared. Differentiation of corneal lesions as primary or secondary is of great importance, for both the diagnostic approach and the treatment of corneal diseases.
> REFERENCES
1. Michel SG, Comparative Anatomy of Domestic Mammals, Thessaloniki, Greece, 1975.
2. Samuelson DA. Ophthalmic Anatomy. In: Veterinary Ophthalmology, Kirk NG. (ed). 4th edn. Blackwell Publishing: Ames Iowa, 2007, 49-60.
3. Gum GG, Gelatt KN, Esson DW. Physiology of the eye. In: Veterinary Ophthalmology, Kirk NG (ed). 4th edn. Blackwell Publishing: Ames Iowa, 2007, 149-182.
4. Gilger BC, Whitley RD, McLaughlin SA, Wright JC, Drane JW. Canine corneal thickness measured by ultrasonic pachymetry. Am J Vet Res 1991, 52: 1570- 1572.
5. Schoster JV, Wickman L, Stuhr C. The use of ultrasonic pachymetry and computer enhancement to illustrate the collective corneal thickness profiles of 25 cats. Vet Comp Ophthalmol 1995, 5: 68-73.
6. Lucarelli M, Dartt DA, Cook B, Bradley N. The lacrimal system. In: Adler’s Physiology of the Eye: Clinical Application. Kaufman P, Alm A, (eds). St Louis: Mosby, 2003, pp. 30-43.
7. Gilger BC. Diseases and Surgery of the Canine Cornea and Sclera. In: Veterinary Ophthalmology, Kirk NG (ed). 4th edn. Blackwell Publishing: Ames Iowa, 2007, pp. 690-752.
8. Macdonald JM, Geroski DH, Edelhauser HF. Effect of inflammation on the corneal endothelial pump and barrier. Curr Eye Res 1987, 6: 1125-1132.
9. Curtis R, Barnett KC. The ocular lesions of infectious canine hepatitis. J Sm Anim Pract 1973, 14: 375-389.
10. Martin C, Dice P. Corneal endothelial dystrophy in the dog. J Am Anim Hosp Assoc 1982, 18: 327-336.
11. Brooks D, Samuelson D, Smith P. Corneal endothelial cell degeneration in a German Shepherd dog. J Sm Anim Pract 1990, 31: 32-35.
12. Gwin RM, Warren JK, Samuelson DA, Gum GG. Effects of phacoemulsification and extracapsular lens removal on corneal thickness and endothelial call density in the dog. Invest Ophthalmol Vis Sci 1983, 24: 227-236.
13. Slatter D. Corneal and Sclera. In: Fundamentals of Veterinary Ophthalmology, Slatter D (ed). 2nd edn. WB. Saunders: Philadelphia, 1990, pp. 257-303.
14. Featherstone HJ, Franklin VJ, Sansom J. Feline corneal sequestrum: laboratory analysis of ocular samples from 12 cats. Vet Ophthalmol 2004, 7: 229-238.
15. Liapis IK. Corneal ulcer. Why, When, How. Abstracts of Congress on Companion Animal Practice. Athens, 2007, p. 50-54.
16. Cook CS. Ocular Embryology and Congenital Malformations. In: Veterinary Ophthalmology, Kirk NG (ed). 4th edn. Blackwell Publishing: Ames Iowa, 2007, 3-36.
17. Fischer CA, Lindley DM, Carlton WC, Van Hecke H. Tumors of the cornea and sclera. In: Ocular Tumors in Animals and Humans, Peiffer RL, Simons KB (eds). Iowa State Press: Ames Iowa, 2002, pp. 149-202.
18. Stiles J, Townsent WM. Feline Ophthalmology. In: Veterinary Ophthalmology, Kirk NG (ed). 4th edn. Blackwell Publishing: Ames Iowa, 2007, pp. 1095-1164.
19. Morgan RV, Abrams KL, Kern TJ. Feline eosonophilic keratitis: A retrospective study of 54 cases (1989-1994). Prog Vet Comp Ophthalmol 1996, 6:131-134.