Panagiota Karamichali DVM, MSc, Kiriaki Ftergioti DVM, George Kazakos DVM, PhD
Companion Animal Clinic, School of Veterinary Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
MeSH keywords:
anesthesia, deep sedation, rabbits, rodents
Abstract
In this report the normal particular characteristics of each species are explained as well as the perioperative risks that may arise. Furthermore, the preanaesthetic management, potential anaesthetic protocols, and the selection of the appropriate anaesthetic regimen for the induction and maintenance of anaesthesia are also described and finally the article is concluded with the principles of postoperative care. Only drug substances that are available in the Greek market to the veterinary clinicians are reported. The aim of this study is to minimise complications that may prove fatal during anaesthesia in a companion animal clinic.
Introduction
In Greece, common small pet mammals include rabbits (family Leporidae) and species of the order Rodentia (mouse, rat, hamster, gerbils, guinea pig, chinchilla). Rabbits and rodents are frequently presented with severe complications and death during anaesthesia, forcing owners as well as veterinarians to avoid it completely. In fact, even though the mortality rate is not particularly high, it is higher compared to dogs and cats (Brodbelt 2009).
Anatomical and physiological features which affect anaesthesia
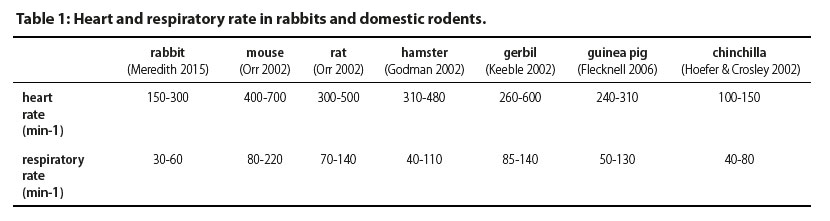
In Table 1, some physiological parameters in rabbits and domestic rodents are presented. An important feature that should be taken into account during the perioperative period is the relatively increased body surface area to body weight ratio, which can be a predisposing factor to hypothermia during anaesthesia. Moreover, the high metabolic rate in combination with the limited glycogen stores can lead to hypoglycaemia (Flecknell 2006). Additionally, parenteral medications have faster onset of action but shorter duration. In case of apnoea, treatment must be immediate. Vital tissues (such as the central nervous system) are quickly rendered hypoxic, due to the high metabolic rate and increased oxygen consumption (Quesenberry & Carpenter 2012).
Catheterisation of a peripheral vein and tracheal intubation can be challenging due to anatomical features and size of these animals (difficulty in visualising the larynx, laryngospasm), therefore special and appropriately sized equipment is required (Harkness et al. 2010).
The required anaesthetic doses are usually lower than reported in studies, in which healthier animals, more accustomed to handling have been included (Harkness et al. 2010). The presence of comorbidities, most of which are asymptomatic (e.g. pasteurellosis, sepsis, pulmonary neoplasia, uterine adenocarcinoma) may affect respiratory system function during anaesthesia.
The sudden reactions of rabbits to poor handling may cause spinal fractures, whereas severe stress may cause cardiac arrhythmias due to catecholamine release (Cantwell 2001). In order to minimise stress in the preanaesthetic ward, cages are required to be away from visual and auditory stimuli. Furthermore, similar species should be housed together, and handling should be minimal and limited to experienced staff.
The prolonged period of preanaesthetic fasting, increased surgical time, severe stress due to pain and change in the environment, may predispose to postoperative ileus. Tympany due to stasis of gastrointestinal content decreases the diaphragmatic contractility and increases intra- abdominal pressure, with potential sequelae on the respiratory and cardiovascular system (Flecknell 2016).
Body weight should be estimated accurately, especially in very small animal species (Harkness et al. 2010). In rodents, especially mice and rats, the subcutaneous and intraperitoneal route are preferred for the administration of fluids, but not for fluids containing dextrose.
Preoperative fasting
In rabbits and domestic rodents, preoperative fasting is not applied due to fast gastric emptying and the potential for gastrointestinal stasis. Therefore, free access is granted to food and water until premedication (Flecknell 2006). Moreover, fasting depletes glycogen stores, causing hypoglycaemia, which may be a primary cause for postoperative ileus.
Rodents do not vomit, therefore preoperative fasting is not required. However, guinea pigs usually store food in the caudal part of the oral cavity, therefore recommendations include fasting of 2-3 hours prior to surgery and removal of food remnants with a cotton bud after induction of anaesthesia (Horn et al. 2013).
Regarding large-breed and obese rabbits, preoperative fasting of one hour may reduce the volume of the stomach and caecum, which may theoretically compress the limited thoracic cavity. In practice however, this does not change gastrointestinal volume sufficiently, in clinical practice (Quesenberry & Carpenter 2012).
Premedication and sedation
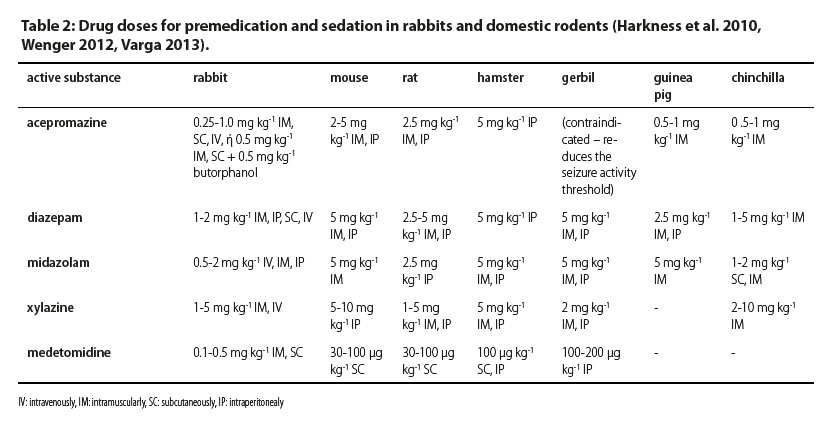
In Table 2 drug doses used in premedication and sedation in rabbits and domestic rodents are shown.
An important factor that should be considered during the selection of premedication agents is rapid recovery. Prolonged recovery time may cause gastrointestinal problems, hepatic lipidosis and hypoglycaemia (Bonath et al. 1982). For this reason, drugs with a wide “safety window” or with an antagonist that allows for reversal of their effect (e.g. medetomidine-atipamezole) should be selected. Moreover, a better result can be accomplished with combinations of drugs. The combination of drugs has an additive effect on accomplishing sufficient sedation and minimizes side effects (Richardson & Flecknell 2005).
Anticholinergic drugs (atropine, glycopyrrolate)
Anticholinergic drugs inhibit parasympathetic nervous system activity and cause tachycardia, reduced bronchial secretions and mydriasis. They are indicated in surgical procedures that cause vagal nerve stimulation due to surgical manipulations (visceral handling, endotracheal intubation), as well as in combination with opioids such as fentanyl that may result in bradycardia (Flecknell 2016).
The administration of anticholinergic agents is recommended in guinea pigs, considering that their respiratory secretions are viscous and plentiful, regardless of whether the animal has been intubated, a mask is used or it is temporarily rendered immobile (Cantwell 2001). Atropine is relatively ineffective in rabbits, due to the presence of atropinesterases in their blood (AtrE), resulting in increased doses of the drug being required (Harrison et al. 2006). Glycopyrrolate is preferred, which is not available in the Greek market. It is worthy of note that the administration of atropine with α-2 adrenergic agonists is not contraindicated in these animals, in contrast with carnivore companion animals (Alibhai et al. 1996).
Benzodiazepines (diazepam, midazolam)
Benzodiazepines are mild sedative and anxiolytic drugs with muscle relaxant action. They have no analgesic properties. They cause mild depression of the cardiovascular and respiratory system and have short duration of action (30-60 minutes).
Midazolam administered intramuscularly or intravenously in rabbits and domestic rodents may cause sufficient sedation for diagnostic procedures, such as radiography and ultrasonography (Johnson- Delaney 2010). When it is combined in lower doses with opioids (e.g. butorphanol) or α-2 agonists (e.g. dexmedetomidine), the resulting sedation is enhanced (Cantwell 2001, Boehm et al. 2010, Schernthaner et al. 2011). Recently intranasal administration of midazolam has also been reported, in combination with α-2 agonists and opioids, for chemical restrain in rabbits (Santangelo et al. 2016).
Diazepam can be administered orally or intravenously. Intravenous injections can result in vascular injury (Flecknell 2009; Papich 2016). Intramuscular and subcutaneous injection is irritating to tissues due to the high osmolality of the solution (Quesenberry & Carpenter 2012).
Flumazenil reverses the effect of benzodiazepines, but due to its shorter half-life the sedative effect of benzodiazepines may return after it has been metabolised (Barr 2007).
Phenothiazines (acepromazine)
Acepromazine has been used in rabbits and domestic rodents ensuring light sedation without analgesia. It can be administered via all routes (subcutaneous, intramuscular, intravenous, oral), in order to reduce the doses of the anaesthetic drugs. All phenothiazines, in general, cause hypothermia and mild hypotension due to peripheral vasodilation, and therefore, they are contraindicated in the presence of hypovolemia, anaemia or shock. Moreover, acepromazine reduces tear production in rabbits resulting in severe corneal and conjunctival disorders (Ghaffari et al. 2009). Its use is contraindicated in gerbils because of lowering the seizure threshold (Quesenberry & Carpenter 2012).
α-2 Adrenergic agonists (xylazine, medetomidine, dexmedetomidine)
α-2Agonists cause deep sedation and simultaneously have analgesic and muscle relaxant effects. Combined with ketamine, they are effective for surgical anaesthesia. Oxygen administration is recommended in all cases (Quesenberry & Carpenter 2012). They characteristically reduce intestinal motility, causing prolonged gastrointestinal transit time. α-2 Agonists also negatively affect thermoregulation in rats, rabbits and guinea pigs (Szreder 1993). One of the most important advantages of medetomidine is the absence of perioperative arrhythmias in contrast with xylazine. Particular care must be taken when administering dexmedetomidine in rats, due to increased incidence of urethral obstruction following recovery from anaesthesia, and high mortality rates (Cagle et al. 2017). Reduction in systemic arterial pressure, heart rate, myocardial contractility and cardiac output are severe side effects of the α-2 adrenergic agonists (Suckow et al. 2012).
The combination of medetomidine-midazolam- fentanyl has been successfully used in chinchillas, guinea pigs, rats, and hamsters. The main advantage of this combination is the full reversal of anaesthesia with atipamezol, flumazenil and naloxone, respectively, therefore reducing recovery time and the risk of hypothermia and hypoglycaemia. However, this combination requires endotracheal intubation and monitoring of respiratory function (Henke et al. 2005, Baumgartner et al. 2010). Mild peripheral vasoconstriction, impeding the insertion of an intravenous catheter is observed with the administration of medetomidine. Atipamezole, at 10-20% of the preanaesthetic dose of medetomidine can reduce the undesirable effects without reversal of anaesthesia and analgesia (Carpenter 2005). Intramuscular injection of medetomidine-midazolam- fentanyl in chinchillas provides anaesthesia for 1.5 hours, however the cardiac and respiratory rate are reduced, and recovery can be prolonged if anaesthesia is not reversed.
Xylazine in combination with ketamine, and detomidine alone or in combination with ketamine- diazepam, administered in repeated doses, are associated with fibrosis and necrosis of myocardium in rabbits (Hurley et al. 1994).
Induction and maintenance of anaesthesia
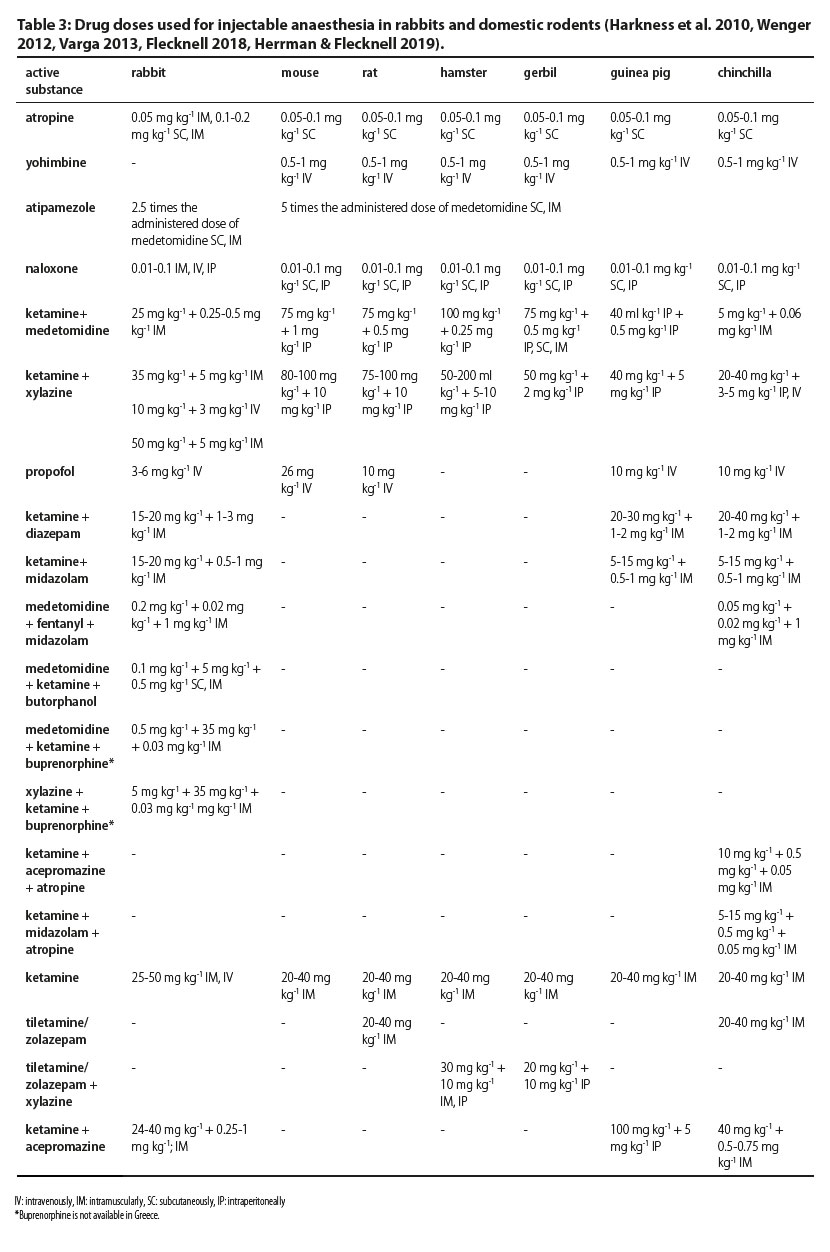
In Table 3 active substances and combinations used in injectable anaesthesia of rabbits and domestic rodents are shown.
Induction of anaesthesia can be accomplished with injectable or inhalational anaesthetics. The exclusive use of inhalational anaesthetics is not always effective and may be proved harmful. This occurs because when these animals are exclusively exposed to inhalational anaesthetics, even in small concentrations, they become stressed and hold their breath for up to 2 minutes. Severe stress results in the release of catecholamines and inhalational anaesthetics sensitise the myocardium to the effect of the latter, which may lead to cardiac arrest (Flecknell 2006).
Anaesthetic circuits for maintenance of anaesthesia should have reduced dead space and low resistance, e.g. Bain or Ayre’s T-piece (Wenger 2012). Inhalational anaesthesia is the first choice for rodent anaesthesia. However, endotracheal intubation is challenging in these species (Longley 2008), therefore anaesthesia is often maintained with a facemask, connected to the anaesthetic circuit (Wenger 2012). Moreover, protocols of total intravenous anaesthesia can be used, or anaesthesia can be enhanced or maintained with inhaled anaesthetics after induction with injectable drugs (Longley 2008).
In guinea pigs and chinchillas, endotracheal intubation is impeded by the attachment of the soft palate to the base of the tongue, which forms palatoglossal cavities, rich in vascular supply, that can easily be injured (Wenger 2012).
Intubation in rabbits is recommended in all anaesthetic procedures except for very short ones. For intubation, a blind technique can be used (auscultating for respiratory sounds) or with visualisation of the larynx with laryngoscope or endoscope. In both techniques, the rabbit is placed in sternal recumbence with the head extended to facilitate intubation. Topical lidocaine on the larynx is applied to avoid laryngospasm during intubation. Especially in rats, an easy and reliable techniques has been described, with the use of a Teflon intravenous catheter 22G, in which the end of the metal guide is cut. The rat is restrained, and a suture is placed around the upper incisors to facilitate mouth opening. Then with a light source the catheter is introduced with rotating moves, and by applying pressure on the epiglottis it is advanced toward the trachea (Papastefanou et al. 2014). Alternatively, laryngeal mask can be used in rabbits, for their advantages in ease of intubation technique and requirement for lower concentrations of induction anaesthetic in order to allow intubation (Kazakos et al. 2007). Even though laryngeal masks can be used for artificial ventilation, gastric tympany is still a possibility, especially if maximal inspiratory pressure raises above 14 cmH2O (Bateman et al. 2005).
Inhalational anaesthetics
The commonest inhalational anaesthetics are isoflurane and sevoflurane. The concentrations required for induction range at 3-4.5% for isoflurane and 5-6% for sevoflurane. Gerbils seem to require higher concentrations compared to other rodents (Keeble 2002). In particular, minimum alveolar concentration (MAC) in non-premedicated rabbits is at 2.07% for isoflurane and 2.9-3.2% for sevoflurane. In rabbits premedicated with ketamine at 0.4-1 mg kg-1 hr-1 or lidocaine at 3-6 mg kg-1 hr-1, the MAC is 1.5% for isoflurane and 2.1% for sevoflurane, respectively (Flecknell 2006, Barter & Epstein 2013, Schnellbacher et al. 2013).
Inhalational anaesthetics have no analgesic properties, therefore analgesics need to be simultaneously administered accordingly. Preoxygenation improves perfusion and tissue oxygen saturation, which is particularly useful in patients with pre-existing cardiac or respiratory disease (Longley 2008). Moreover, in order to prevent breath-holding, oxygen should be provided exclusively at first and the concentrations of inhalational anaesthetic should be gradually increased. In guinea pigs breath-holding is so prominent, that an anaesthetic cage cannot be used for induction. Breath-holding can lead to sudden, deep inhalation of the anaesthetic agent followed by cardiac and respiratory arrest (Flecknell et al 1996).
In rabbits, sevoflurane is often preferred for rapid induction, which seems to be fairly well-tolerated and does not sensitise myocardial tissues to the arrhythmogenic effect of catecholamines, if the rabbit becomes stressed (Piriou et al. 2002). In rodents, sevoflurane recovery is smooth. Differences in induction and recovery times compared to isoflurane are insignificant (Preckel et al. 2005). In guinea pigs, isoflurane often causes lacrimation and salivation (Schmitz et al. 2016).
Even though sevoflurane appears to be less irritating to the airways, secretions from the upper respiratory tract may still be increased. It is possible to begin induction by sevoflurane and continue with isoflurane for financial reasons (Allweiler 2010).
Injectable anaesthetics
Propofol
Propofol can be used in rabbits, with or without premedication for intubation. Propofol in these animals can cause apnoea, hypoxia and hypotension. It is of utmost importance to inject it slowly (more than 60 sec per induction bolus), in order to prevent apnoea and to accomplish smooth induction of anaesthesia (Allweiler 2010). However, it is not useful for maintenance of anaesthesia in painful surgeries of long duration, considering that it offers inadequate depth of anaesthesia without analgesia (Wenger 2012). Its use is limited in rodents, because intravenous access in this species is difficult to accomplish. Furthermore, the apnoea caused by propofol can be highly challenging due to the difficulty of administering a bolus slowly to animals of such small size (Flecknell 2006) as well as the difficulty in securing the airway.
Ketamine
ΗKetamine may be administered by the intramuscular, subcutaneous, intraperitoneal and intravenous route and it has a mild analgesic effect (Hedenqvist et al. 2002). When ketamine is injected in combination with medetomidine, xylazine, diazepam in one bolus, it is most effective in induction of anaesthesia in rabbits and rodents (Flecknell 2016). Moreover, in rabbits it can be injected with propofol, a combination with a dose-dependent effect requiring the simultaneous administration of oxygen (Santos et al. 2016).
When administered as the only agent in rodents, ketamine causes immobilisation rather than surgical anaesthesia. In small rodents, the high doses required for ensuring surgical anaesthesia can cause severe respiratory depression. Recovery may be delayed and possibly complicated with hyperactivity and delirium (Flecknell 2016). The intramuscular route has been implicated in self-mutilation in rabbits (Shientag 2011, Wenger 2012).
Ketamine is often combined with medetomidine or xylazine for chemical restrain and surgical anaesthesia of short duration, administered by single injection, usually by the intraperitoneal route. In rabbits, the combination of ketamine-medetomidine facilitates rapid intubation, less use of isoflurane and smaller degrees of heat loss compared to ketamine-midazolam but is usually implicated in laryngospasm (Grint et al. 2008). The combination of ketamine-medetomidine also provides better depth of anaesthesia and longer duration in healthy rabbits than the combination of medetomidine-midazolam- fentanyl. However, it can lead to severe reduction in arterial pH and partial pressure of oxygen, resulting in post-surgical apnoea. It is also responsible for reduction in body temperature by 4-4.6°C in mice, rats and hamsters (Cruz et al. 1998, Longley 2008).
The effect of α-2 adrenergic receptor agonists combined with ketamine appears to be inadequate in sedating guinea pigs, which may not be sufficiently anaesthetised (Quesenberry & Carpenter 2012). In any case, induction of anaesthesia with ketamine-xylazine is required prior to administration of any inhalational anaesthetic.
In order to manage increased bronchial secretions and hypersalivation, the administration of atropine (or glycopyrrolate) is sometimes necessary (Flecknell 2016).
Tiletamine-Zolazepam
The combination of tiletamine-zolazepam is available in Greece under the trade name Zoletil. Tiletamine has a similar chemical structure to ketamine. Zolazepam is a benzodiazepine pharmacologically similar to diazepam. Zolazepam enhances the effect of tiletamine on the central nervous system. It also prevents seizure activity due to tiletamine and improves muscular relaxation and anaesthetic recovery.
In rats, it is administered in combination with xylazine causing cardiovascular depression, and in combination with butorphanol with minimal depression of the cardiovascular and respiratory system (Wilson et al. 1993).
It is contraindicated in rabbits due to potential nephrotoxicity (Doerning et al. 1992).
Recovery and postoperative care
Supportive measures and monitoring should be continued during the postoperative period. Room temperature should be preserved initially at around 35°C and then as consciousness is regained temperatures can be reduced to 26- 28°C (Caro et al. 2013). Warm and comfortable bedding should be provided. Wood shavings are inappropriate but pellets or dry bedding material are recommended for the initial postoperative recovery period. Afterward, when consciousness has been regained, the animal can be moved to a cage or pen area with pieces of newspaper (mice, rats) or good quality hay or straw (chinchillas, guinea pigs). Sand baths for chinchillas should be removed until the animal has fully recovered, otherwise ocular injuries or inhalation of dust may occur (Cantwell 2001, Flecknel 2006).
In all species parenteral administration of warm (37°C) dextrose solution and/or normal saline (1-2 ml NaCl 0.9% per 100 g of body weight SC) is recommended at the end of surgery, as intravenous fluid support for the postoperative period (Flecknel 2006, Hoff et al. 2006).
Food uptake should be encouraged as soon as possible after recovery. Mice and rats may prefer soft food, whereas rabbits and guinea pigs may consume pellets, grass or special commercial liquefied diet for animals with increased postoperative nutritional needs. In order to prevent postoperative ileus, prokinetic drugs should be administered (metoclopramide 0.2-1 mg kg-1, PO, SC, or IM, every12 hours). In rats, non-steroidal anti-inflammatory drugs are also indirectly effective in reducing postoperative stress and pain. Studies have in fact indicated that the repeated administration of non-steroidal anti-inflammatory drugs, such as meloxicam, provides more efficient analgesia than opioids (Cooper et al. 2009, Bourque et al. 2010). It is especially important that these animals are hospitalised away from the sight, sound, and smell of predators, such as cats and dogs.
Conflict of interest
The authors declare no conflicts of interests.
References
- Alibhai, HK, Clarke KW, Lee YH, Thompson J (1996) Cardiopulmonary effects of combinations of medetomidine hydrochloride and atropine sulphate in dogs. Vet Rec 138, 11–13.
- Allweiler S, Leach MC, Flecknell PA (2010) The use of propofol and sevoflurane for surgical anaesthesia in New Zealand White rabbits. Lab Anim 44, 113–117.
- Bateman L, Ludders JW, Gleed RD, Erb HN (2005) Comparison between facemask and laryngeal mask airway in rabbits during isoflurane anesthesia. Vet Anaesth Analg 32, 280-288.
- Barr J (2007) Reversal Agents: Naloxone and Flumazenil. Complications in Anesthesia, 2nd ed, (Saunders Elsevier, Philadelphia) pp. 128- 130.
- Barter LS, Epstein SE (2013) Cardiopulmonary effects of three concentrations of isoflurane with or without mechanical ventilation and supramaximal noxious stimulation in New Zealand white rabbits. Am J Vet Res 74, 1274-1280.
- Baumgartner C, Bollerhey M, Ebner J, Laacke-Singer L, Schuster T, Erhardt W (2010) Effects of ketamine-xylazine intravenous bolus injection on cardiovascular function in rabbits. Can J Vet Res, 74, 200-208.
- Boehm Β, Carney Ε, Tallarida Ρ, Wilson Ρ (2010) Midazolam enhances the analgesic properties of dexmedetomidine in the rat, Vet Anesth Analg 37, 550-556.
- Bourque SL, Adams MA, Nakatsu K (2010) Comparison of buprenorphine and meloxicam for post-surgical analgesia in rats: effects on body weight, locomotor activity. J Am Assoc Lab Anim Sci 49, 617–622.
- Brodbeld D (2009) Perioperative mortality in small animal anaesthesia. Vet J 182, 152-160.
- Cagle L, Franzi L, Epstein S, Kass P, Last J, Kenyon N (2017) Injectable Anesthesia for Mice: Combined Effects of Dexmedetomidine, Tiletamine-Zolazepam, and Butorphanol, Anesthesiol Res Pract, ID: 9161040. pp.1-7.
- Cantwell SL (2001) Ferret, rabbit, and rodent anesthesia. Veterinary Clin North Am Exotic Animal Practice 4, 169-191.
- Caro AC, Hankenson FC, Marx JO (2013) Comparison of thermoregulatory devices used during anesthesia of C57BL/6 mice and correlations between body temperature and physiologic parameters. J Am Assoc Lab Anim Sci 52, 577-583.
- Carpenter JW (2005) Ferrets. Exotic Animal Formulary. 3rd ed. Elsevier Health Science, (London, United Kingdom) pp. 447–476.
- Cooper C, Metcalf-Pate K, Barat K, Cook J, Scorpio D (2009) Comparison of Side Effects between Buprenorphine and Meloxicam Used Postoperatively in Dutch Belted Rabbits (Oryctolagus cuniculus). J Am Assoc Lab Anim Sci 48, 279–285.
- Crotaz I R (2010). Initial feasibility investigation of the v-gel airway: an anatomically 9 / 10 designed supraglottic airway device for use in companion animal veterinary anaesthesia. Vet Anaesth Analg 37, 579-580.
- Cruz F and Junquera J (1993) The immobility response elicited by clamping, bandaging and grasping in the Mongolian gerbil (Meriones unguiculatus). Behav Brain Res 54, 165-169.
- Doerning BJ, Brammer DW, Chrisp CE, Rush HG (1992) Nephrotoxicity of tiletamine in New Zealand white rabbits. Lab Anim Science 42, 267-269.
- Flecknell P (2006) Anaesthesia and perioperative care. In: Meredith A, Flecknell P, editors. BSAVA Manual of Rabbit Medicine and Surgery. Quedgeley, UK, pp. 154–65.
- Flecknell P (2016) Laboratory Animal Anaesthesia, 4th ed., Academic Press, (Newcastle, UK)
- Fox L, Snyder L, Mans C (2016) Comparison of dexmedetomidine– ketamine versus isoflurane anesthesia in chinchillas (Chinchilla lanigera). J Am Assoc Lab Anim Sci 55, 1–5.
- Flecknell P (2018) Rodent analgesia: Assessment and therapeutics. Vet J 232, 70-77.
- Ghaffari MS, Moghaddassi AP, Bokaie S (2009) Effects of intramuscular acepromazine and diazepam on tear production in rabbits. Vet Rec 164, 147-148.
- Goodman G (2002) Hamster. In: BSAVA manual of exotic pets. (4th edn), BSAVA, Hampshire, pp. 27-33.• Grint NJ, Murison PJ (2008) A comparison of ketamine-midazolam and ketamine-medetomidine combinations for induction of anaesthesia in rabbits. Vet Anaesth Analg 35, 113-121.
- Harkness JE, Turner PV, Woude S, Wheler CL (2010) Harkness and Wagner’s Biology and Medicine of Rabbits and Rodents, 5th edn. Wiley-Blackwell, Iowa, pp. 147-239.
- Hedenqvist P, Orr HE, Roughan JV, Antunes LM, Flecknell PA (2002) Anaesthesia with ketamine/medetomidine in the rabbit: influence of route of administration and the effect of combination with butorphanol. Vet Anaesth Analg 29, 14-19.
- Henke J, Astner S, Brill T, Eissner B, Busch R, Erhardt W (2005) Comparative study of three intramuscular anaesthetic combinations (medetomidine/ketamine, medetomidine/fentanyl/ midazolam and xylazine/ ketamine) in rabbits. Vet Anaesth Analg 32, 261-270.
- Herrmann K and Flecknell P (2019) Retrospective review of anesthetic and analgesic regimens used in animal research proposals. ALTEX - Alternatives to animal experimentation 36, 65-80.
- Hoefer HL, Crossley DA (2002) Chincillas. In: BSAVA manual of exotic pets. (4th edn), BSAVA, Hampshire, pp. 65-75.
- Hoff JB, Dysko R, Kurachi S, Kurachi K (2006) Technique for performance and evaluation of parapharyngeal hypophysectomy in mice. J Am Assoc Lab Anim Sci 45, 57-62.
- Horn C, Kimball B, Wang H, Kaus J, Dienel S, Nagy A, Gathright G, Yates B, Andrews P (2013) Why Can’t Rodents Vomit? A Comparative Behavioral, Anatomical, and Physiological Study, PLoS One 8, 101-171.
- Hurley RJ, Marini RP, Avison DL, Murphy JC, Olin JM, Lipman NS (1994) Evaluation of detomidine anesthetic combinations in the rabbit. Lab Anim Science 5, 472-478.
- Johnson-Delaney C (2016) Ferret Medicine and Surgery, CRC Press, Washington, pp. 312-407.
- Kazakos GM, Anagnostou T, Savvas I, Raptopoulos D, Psalla D, Kazakou IM (2007) Use of the laryngeal mask airway in rabbits: placement and efficacy. Lab Anim 36, 29-34.
- Keeble E (2002) Gerbils. In: Meredith A and Redrobe S (eds), BSAVA Manual of Exotic Pets (4th edn), BSAVA. pp. 34-46.
- Kohn DF, Martin TE, Foley PL, Morris TH, M Swindle M, Vogler GA, Wixson S (2007) Guidelines for the Assessment and Management of Pain in Rodents and Rabbits, J Am Ass Lab Anim Science 46, 97-108.
- Longley L (2008) Anaesthesia of Exotic Pets. Elsevier, Philadelphia.
- Orr HE (2002) Rats and Mice. In: BSAVA manual of exotic pets. (4th edn), BSAVA, Hampshire, pp. 16-17.
- Papastefanou A, Balafas E, Duracevic S, Kostomitsopoulos N (2014) A simple method of endotracheal intubation in mice. Arch Biol Sci 66, 241-244.
- Papich MG (2016) Diazepam. Saunders Handbook of Veterinary Drugs, pp. 228–229.
- Phaneuf L R, Barker S, Groleau M A and Turner P V (2006) Tracheal injury after endotracheal intubation and anaesthesia in rabbits. J Am Assoc Lab Anim Sci 45, 67-72.
- Piriou V, Chiari P, Lhuillier F, Bastien O, Loufoua J, Raisky O, David JS, Ovize M, Lehot JJ (2002) Pharmacological preconditioning: comparison of desflurane, sevoflurane, isoflurane and halothane in rabbit myocardium 89, 486-491.
- Preckel B, Bolten J (2005) Pharmacology of modern volatile anaesthetics, Best Practice and Research. Clin Anaesthesiol 19, 331–348.
- Quesenberry K and Carpenter J (2012) Ferrets, Rabbits, and Rodents, Clinical Medicine and Surgery, 3rd ed, Elsevier, Missouri, pp. 429-451.
- Quiroz-Padilla MF, Guillazo-Blanch G, Sanchez MY, Dominguez- Sanchez MA, Gomez RM (2018) Effects of Excitotoxic Lesion with Inhaled Anesthetics on Nervous System Cells of Rodents. Curr Pharm 24, 4-14.
- Richardson CA & Flecknell PA (2005) Anaesthesia and Postoperative Analgesia following Experimental Surgery in Laboratory Rodents: Are we Making Progress? Alternatives to Laboratory Animals 33, 119–127.
- Santangelo B, Micieli F, Marino F, Reynaud F, Cassandro P, Carfora A, Petrella R, Borriello R, Cataldi M, Vesce G (2016) Plasma concentrations and sedative effects of a dexmedetomidine, midazolam, and butorphanol combination after transnasal administration in healthy rabbits. J Vet Pharmacol Ther 39, 408-411.
- Santos M, Viñuela A, Vela A, Tendillo FJ (2016) Single-syringe ketamine-propofol for induction of anaesthesia in rabbits. Vet Anaesth Analg 43, 561-565.
- Schernthaner A, Lendl C, Hartmann K, Pragst I, Preissel AK, Henke J (2011) Medetomidine/ midazolam/ ketamine anaesthesia in ferrets: effects on cardiorespiratory parameters and evaluation of plasma drug concentrations, J Vet Anesth Analg 38, 439-450.
- Schmitz S, Tacke S, Guth B, Henke J (2016). Comparison of Physiological Parameters and Anaesthesia Specific Observations during Isoflurane, Ketamine-Xylazine or Medetomidine- Midazolam-Fentanyl Anaesthesia in Male Guinea Pigs. PLoS One 11, e0161258, 1-22.
- Schnellbacher R, Carpenter JW, Mason D, KuKanich B, Hugues Beaufrère H, Boysen C (2013) Effects of lidocaine administration via continuous rate infusion on the minimum alveolar concentration of isoflurane in New Zealand White rabbits (Oryctolagus cuniculus). Am J Vet Res 74, 1377-1384.
- Shientag LJ, Goad M, (2011) Sudden hind limb injuries in two rabbits. Self-mutilation after intramuscular ketamine-related neuronal injury. Lab Anim 21, 212-216.
- Szreder Ζ (1993) Comparison between thermoregulatory effects mediated by alpha 1- and alpha 2-adrenoceptors in normothermic and febrile rabbits. Gen Pharmacol 24, London, UK.
- Suckow MA, Stevens KA, Wilson RP (2012) The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. American College of Laboratory Animal Medicine Series. Academic Press. Elsevier, Netherlands, pp. 36-38.
- Varga M (2013) Textbook of Rabbit Medicine. 2nd Ed, Butterworth- Heinemann, Toronto, pp. 178-203.
- Wenger S (2012) Anesthesia and analgesia in rabbits and rodents. J Exotic Pet Med 21, 7–16.
- Wilson R, Zagon I, Larach D, Lang M (1993) Cardiovascular and respiratory effects of tiletamine-zolazepam. Pharm Bioch Behavior 44, 1-8.
Corresponding author:
Panagiota Karamichali
e-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.



