Kiriaki Pavlidou DVM, PhD, Ioannis Savvas DVM, PhD
Companion Animal Clinic, School of Veterinary Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
MeSH keywords:
diaphragm, dog, Intensive Care Unit
Abstract
Patients in the Intensive Care Unit (ICU) are frequently exhibit marked alterations in organ function. The ICU is fully equipped to monitor the critically ill patient and the respiratory function is one of the vital functions monitored. However, the respiratory muscles are poorly monitored in the ICU, although there is evidence that respiratory muscle dysfunction develops in critically ill patients and can cause respiratory failure. There are many available tools to monitor the respiratory muscle function both in humans and animals. The best indicator of diaphragmatic contractility seems to be the measurement of trans-diaphragmatic pressure (Pdi) in medical and veterinary clinical practice. Trans-diaphragmatic pressure is the difference between the intra-gastric pressure (Pgast) and the intra-oesophageal pressure (Poes). The measurements are made using two air-filled balloon catheters, which are placed in the midthird of the oesophagus and into the stomach. Although the technique of Pdi measurement has been investigated in normal healthy dogs under general anaesthesia with the application of Mueller’s manoeuvre, the diaphragmatic function has not been studied in critically ill dogs in a non-fatigued diaphragm in a clinical setting. Twenty-seven client-owned dogs, status ASA II-IV, were enrolled in this cohort study. Trans-diaphragmatic pressure was measured with the application of the Mueller’s manoeuvre within the first 24 hours of hospitalization in the ICU under general anaesthesia. The mean±standard deviation of Pdi was 11.2±5.7 mmHg and that of blood lactate concentration was 2.4±1.2 mmol L-1. In conclusion, the technique of Pdi measurement with balloon catheters can be successfully applied in dogs in the ICU. Trans-diaphragmatic pressure measurement can be a useful tool for the assessment of diaphragmatic function in critically ill dogs at admission and/or during their hospitalization in the ICU and its application seems to be feasible.
Introduction
Patients in the Intensive Care Unit (ICU) are frequently exhibit severe alterations in organ function. Monitoring is defined as a real time continuous evaluation of the physiological functions of a patient in order to guide therapeutic management (Heunks et al. 2015). The ICU-environment is fully equipped to monitor the critically ill patient. Cardiopulmonary, renal and gastrointestinal function are usually monitored closely and continuously in ICU patients (Doorduin et al. 2013).
A recent large prospective cohort study in human patients (Canet & Gallart 2013) showed high mortality in the ICU after major surgery and underlined the role of the postoperative pulmonary complications. Respiration is a vital function of the body and its monitoring is one of the cornerstones in medical and veterinary clinical practice, during anaesthesia and in the ICU. Concerning monitoring of the respiratory system, there is a lack of a clear definition regarding which signals and variables should be prioritized. There is evidence that respiratory muscle dysfunction develops in critically ill patients and can cause respiratory failure, but the respiratory muscles are poorly monitored in the ICU (Laghi et al. 2003, Hermans et al. 2010, Jaber et al. 2011). Respiratory muscle activity is routinely monitored in very few human ICUs. This may be associated with the limited knowledge on the effects of a critical illness on respiratory muscles, the limited availability of tools to monitor the respiratory muscle function and the concept that close monitoring of respiratory muscles function in critically ill patients is of no clinical importance (Doorduin et al. 2013).
In the veterinary literature, there are studies on animal models which show that the diaphragm is extremely sensitive to shock in general and to sepsis in particular (Aubier et al. 1981, Hussain et al. 1985, Hussain et al. 1988), and diaphragmatic fatigue has already been recognized in most chronic respiratory problems in clinical practice in dogs. However, there is no data about the evaluation of diaphragmatic dysfunction in critically ill dogs in the ICU. Moreover, no clinical study can be found in the literature on the assessment of diaphragmatic contractility in critically ill dogs in the ICU.
Early clinical detection of peripheral or respiratory muscle weakness is difficult. Patients with peripheral muscle weakness exhibit this weakness when they recover from the acute phase of an illness. Symptoms of muscle paralysis can be seen in such cases. The most widely used tool to assess peripheral muscle strength in humans is the Medical Research Council examination, which tests the strength of three muscle groups in each limb (Callahan & Supinski 2009). Techniques to monitor the respiratory system during hospitalization in the ICU have been developed over the years. There are many available tools to monitor the respiratory muscle function both in humans and in animals. Clinically, respiratory muscle weakness can be assumed when patients are difficult to wean from mechanical ventilation. Pressure-volume (detection of lung recruitment, compliance and overdistention) and flow-volume (diagnosis of type of respiratory disease) recordings, capnography (information about respiratory rate and rhythm, cardiac output, dead space calculations), oesophageal manometry, respiratory plethysmography, electromyography, ultrasonography, circulatory biomarkers, computed tomography (CT) and magnetic resonance imaging (MRI) are some techniques that can be used for the assessment of respiratory function (Doorduin et al. 2013). However, in medical and veterinary clinical practice, the best indicator of diaphragmatic contractility seems to be the measurement of trans-diaphragmatic pressure (Pdi) (Benditt 2005).
Pdi is the difference between the intra-gastric pressure (Pgast) and the intra-oesophageal pressure (Poes). The measurements are made with air-filled balloon catheters, which are placed in the midthird of the oesophagus and into the stomach (Watson et al. 2001, Benditt 2005, Pavlidou et al. 2014).
Up to today, the measurement of Pdi as a technique to evaluate the diaphragmatic fatigue has been studied in a fatigued-paralyzed diaphragm by means of electrical stimulation of the phrenic nerves and without application of the Mueller’s manoeuvre. As respiratory muscle fatigue is defined any inability of the respiratory muscles to continue to generate sufficient pressure to maintain alveolar ventilation and it is reversible in rest (Hubmayr et al. 1990). Although the technique of Pdi measurement has been investigated in normal healthy dogs under general anaesthesia with the application of Mueller’s manoeuvre (Pavlidou et al. 2013, Pavlidou et al. 2014), the diaphragmatic function has not been studied in critically ill dogs in a non-fatigued diaphragm in a clinical setting.
Pathological conditions such as respiratory muscle weakness and/or diaphragmatic fatigue are well associated with abnormal Pdi. According to the literature, the maximum Pdi (Pdi max) is taken into account for the assessment of the diaphragmatic muscle weakness (Hubmayr et al. 1990). Therefore, Pdi max is obtained during the maximal inspiratory effort with the application of the Mueller’s manoeuvre, as it has been described in previous studies (Pavlidou et al. 2013, Pavlidou et al. 2014).
In critically ill dogs, the respiratory system is usually affected (Hussain et al. 1985), but there is also a lack of knowledge and application of tools to monitor respiratory muscle function in the ICU. The aim of this clinical study was to report on the application and the measurement of Pdi in critically ill dogs in the ICU as an indicator of diaphragmatic contractility.
Materials and Methods
This study was approved by the Ethics Committee of the Aristotle University of Thessaloniki (2016-050-0503-8401). All the dog owners were informed in detail about the study protocol and a signed written consent was obtained. The animals were excluded from the study when the collection of the data was impossible (e.g. ineffective measurement of Pdi). Another exclusion criterion was obesity (nutritional status over 4), which has been shown to decrease diaphragmatic contractility (Ora et al. 2011).
Twenty-seven client-owned dogs, status ASA II-IV, were enrolled in this clinical study. For each dog, age, breed, sex, body weight and clinical diagnosis of illness were recorded within the first 24 hours following ICU admission; mentation score was assessed at admission in order to estimate the true baseline mental status before the administration of any analgesia or anaesthesia. The dogs were allocated to five groups: peritonitis/ intra-abdominal surgery, intra-thoracic surgery, respiratory disease, neurologic disease and neoplasia. A thorough clinical examination was performed and values for certain respiratory parameters regarding the evaluation of the respiratory function were obtained on admission of the dog in the ICU (Hayes et al. 2010). Arterial blood gases analysis gave information about the pH, oxygen partial pressure (PaO2), carbon dioxide partial pressure (PaCO2) and the ratio oxygen partial pressure/fraction of inspired oxygen (PaO2/FiO2). Moreover, the blood concentration of lactate as an index of the severity of a critical case was measured on admission.
The Pdi was measured with the application of the Mueller’s manoeuvre, during the first 24 hours of the animal hospitalisation in the ICU, under the same plane of anaesthesia in all animals. At a surgical anaesthetic level (lack of reflexes, adequate muscle relaxation, lack of response to surgical stimulation), two 90 cm long oesophageal balloon catheters with guide wires (Esophageal Balloon Catheter Set; CooperSurgical Company, CT, USA) were introduced orally. Using the landmarks that have been previously described (Waterman & Hashim 1991, Pavlidou et al. 2014), the balloon of the first catheter was introduced into the stomach for the measurement of Pgast and the balloon of the second catheter was positioned in the mid-third of the oesophagus for the measurement of Poes. The correct positioning of the balloon catheters was confirmed by the observation of positive and negative pressure tracings of Pgast and Poes, respectively, on a computer screen. The catheters were secured in place by fixing them on the endotracheal tube. The guide wires were removed, the catheters were connected to the pressure transducers and the balloons were inflated with 0.5-1 ml of air. The electrical connections of the transducers were attached to a pressure monitoring device with the appropriate software (Pressure Monitoring system Buzzer-II; Michael Roehrich, Austria) and then to a computer. The pressure transducers were zeroed to the atmospheric pressure prior to each measurement. In order to obtain the maximum Poes, Pgast and Pdi, a modified Mueller’s manoeuvre was applied. In particular, the endotracheal tube was disconnected from the anaesthetic circuit and the distal end of the tube was tightly closed with a thumb during the respiratory pause after the end of expiration, and thus forcing the dog to breath against the obstructed airway (modified Mueller’s manoeuvre) (Pavlidou et al. 2014).
The anaesthetic protocol could not be absolutely standardized for all animals as they were suffering from different clinical conditions. Thus, the anaesthetic protocol was selected in a way to minimally affect the Pdi (Pavlidou et al. 2013). For this reason, the preanesthetic medication differed among the animals, whereas the induction and the maintenance of anaesthesia was the same in all animals. Anaesthesia was induced with propofol (Propofol MCT/LCT, Fresenius, Fresenius Kabi, Greece) intravenously to effect. An initial dose 1-2 mg kg-1 was given followed, if needed, by incremental doses of 0.5 mg kg-1 until endotracheal intubation could be easily performed. Anaesthesia was maintained with isoflurane (Isoflurane, Merial, Italy) in oxygen. All animals were breathing spontaneously. Fresh gas (100% oxygen) flow was delivered at 1,5 L min-1 through a circle rebreathing system.
For the statistical analysis, analysis of variance was used to evaluate any difference of the measured variables among the groups with a computer software (IBM SPSS 24). The level of significance was set at p=0.05.
Results
Twenty-seven dogs (17 males, 10 females) 1-15 (6.6±4) years (mean±standard deviation) old, weighing 3-40 kg (16.7±12.3) were included in the study. The insertion of the balloon catheter was impossible in 15 cases, because the catheter could not pass through the lower oesophageal sphincter. Ten dogs were premedicated with dexmedetomidine (Dexdomitor, Pfizer, Greece) at 175 μg m-2 intramuscularly (IM) alone or in combination with methadone (Synthadon, Le- Vet, The Netherlands) at 0.1 mg kg-1 IM, 5 dogs with acepromazine (Acepromazine, Alfasan, The Netherlands) at 0.05 mg kg-1 IM and methadone at 0.1 mg kg-1 IM, 8 dogs with fentanyl (Fentanyl, Janssen-Cilag, Greece) at 1 μg kg-1 and midazolam (Dormipnol, Viofar, Greece) at 0.5 mg kg-1 intravenously, and finally 4 dogs were not premedicated at all.
Mean±standard deviation of Pdi was 11.2±5.7 mmHg, lactate 2.4±1.2 mmol L-1, PaO2 349±171.7 mmHg, PaCO2 49±14.4 mmHg, and the ratio PaO2/FiO2 352.2±156.4 mmHg. Descriptive statistics for all the above parameters in the five different groups are shown in Table 1.
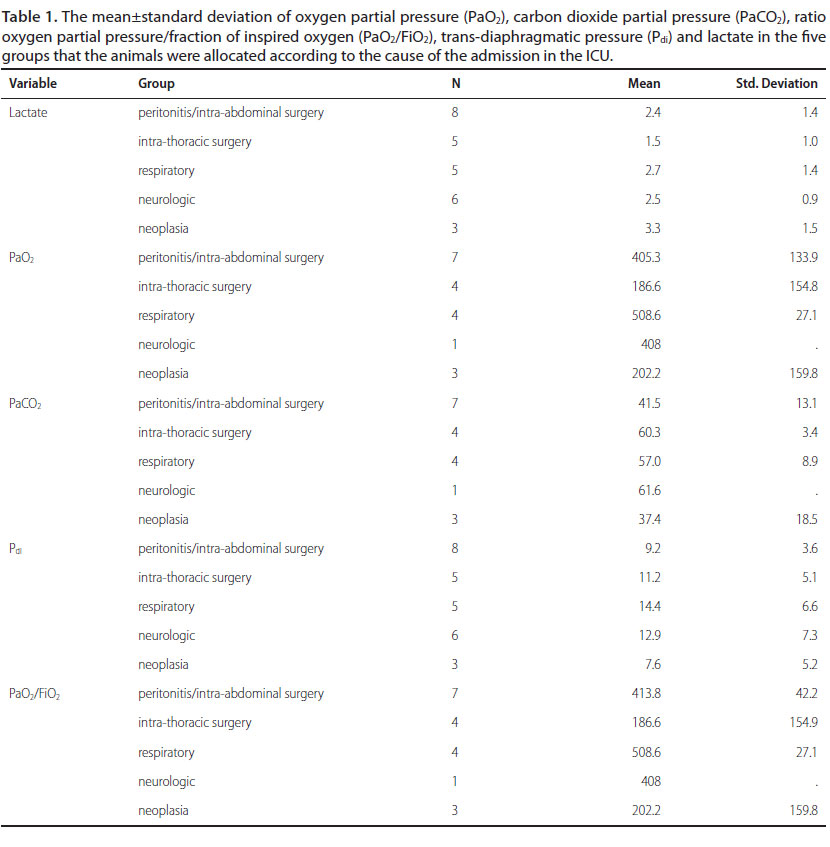
There was statistically significant difference in the PaO2 (p=0.015) and the ratio PaO2/FiO2 (p=0.002) among the five groups. In contrast, there was no statistically significant difference in the Pdi (p=0.368), the PaCO2 (p=0.054) and the lactate concentration (p=0.368) among the groups.
Discussion
The main finding of this study is that the technique of Pdi measurement with the use of balloon catheters can be successfully applied in dogs in the ICU. The respiratory system consists of two parts: the lungs as an exchanging organ and a ventilatory pump. The diaphragm is the main respiratory muscle and it accounts for about 60% of the tidal volume in supine position in humans. Although it has received little attention, its normal function is of great importance for the anaesthetist (Pavlidou et al. 2014). Its dysfunction is usually recognized, especially in humans, when it is not possible to wean from the mechanical ventilation. Therefore, the evaluation of diaphragmatic function is a useful tool in the ICU to assess respiratory failure in critically ill patients.
According to the literature, the main cause for admission in the ICU is respiratory problems, such as pleural effusions and diaphragmatic hernia in humans. Moreover, the majority of pathological conditions influence the respiratory function, and acute respiratory distress syndrome (ARDS) arises as a consequence of severe diseases. Thus, diaphragmatic dysfunction may develop upon admission in the ICU or during the hospitalization (Berger et al. 2016).
In humans, diaphragmatic function is a major determinant that affects not only weaning from the mechanical ventilation in the ICU patients, but also the duration of hospitalization (Jaber et al. 2011). The effect of critical illness on respiratory muscle function is known as “ICU-acquired weakness” and it is induced via many different pathophysiological mechanisms (Kress & Hall 2014). This phenomenon is a major cause of mortality and “long-term” morbidity in ICU-patients, but its true prevalence is not known (Callahan & Supinski 2009).
According to the literature in human medicine, early descriptions of “ICU-acquired weakness” in critically ill patients had been reported by Osler (1915) on neuromuscular dysfunction in patients with sepsis and by Olsen (1956) on peripheral neuropathy in patients in coma. Later, myopathy was described in patients with status asthmaticus by McFarlane & Rosenthal (1977), and polyneuropathy in patients in the ICU by Bolton et al. (1984).
Systemic inflammation, drugs, electrolyte disturbances and immobility have been described as causes of the pathogenesis of the “ICU-acquired weakness” (Jolley et al. 2016). Limb and respiratory muscle weakness are the most common clinical findings in this syndrome. Respiratory muscle weakness is observed as decreased diaphragmatic strength, and consequently ARDS develops (Hermans et al. 2010).
Two patterns of diaphragmatic dysfunction have been described in critically ill patients. Firstly, the diaphragm, as all the other striated muscles, can be involved in the shock-related generalized organ failure and it is observed in many patients in the ICU. According to the second mechanism, diaphragmatic dysfunction can occur during the hospitalization in the ICU, as a consequence of neuromuscular disorder or prolonged mechanical ventilation (Demoule et al. 2013, Demoule et al. 2016).
Ventilatory support remains an essential and life-saving therapy for patients in the ICU with acute respiratory failure. According to Esteban et al. (2000), 40% of critically ill patients in the ICU are mechanically ventilated for a median duration of 5-7 days and 30% of those have problems weaning from the ventilator (Esteban et al. 1995). Respiratory muscle weakness which is caused by mechanical ventilation seems to be a state of respiratory fatigue. Ventilator-induced diaphragmatic dysfunction (VIDD) is defined as a loss of diaphragmatic force-generating capacity because of mechanical ventilation (Berger et al. 2016). This decrease in force-generating capacity is not related to changes in lung volume, abdominal compliance or phrenic nerve function. In contrast, it suggests a primary diaphragm muscle dysfunction which is associated with cell changes. Additionally, diaphragmatic dysfunction may be caused secondary to other causes such as sepsis, administration of systemic corticosteroids and neuromuscular blocking agents (Ochala et al. 2011), multiple organ failure and hypercapnia (acidosis).
According to the above, it would seem essential to evaluate the respiratory function in critically ill dogs. Therefore, investigation of the feasibility of Pdi measurement in the ICU was the main aim of this clinical study.
In the present clinical study, the premedication could not be standardized in all the critically ill patients, because of their different clinical status. This is a limitation of the study, as the different types of preanesthetic medications used may have variably affected the diaphragmatic contractility. However, the protocol for the induction and the maintenance of anaesthesia was the same in all animals. According to a previous study, fentanyl and propofol seem to reduce diaphragmatic contractility, as Pdi values were 12.0±5.9 mmHg and 12.2±3.2 mmHg respectively, in comparison with isoflurane (14.9±4.7 mmHg) in dogs under anaesthesia (Pavlidou et al. 2013). However, the value of Pdi in the present study was lower than the above reference values and this may imply depression of diaphragmatic contractility in critically ill dogs in the ICU.
The PaO2/FiO2 ratio is used as an index of two important respiratory syndromes with high morbidity and mortality: acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) (Rubenfeld et al. 2005, Matthay et al. 2012). A mean value of PaO2/FiO2 less than 300 mmHg indicates ALI and less than 200 mmHg ARDS, both in humans and in animals (Calabro et al. 2013). In this clinical study, the mean±standard deviation of PaO2/FiO2 ratio was less than 300 mmHg only in the group of neoplasia and this is indicative of the occurrence of the ALI syndrome in these animals.
Measurement of lactate blood concentration is considered to be a useful tool in human and veterinary clinical practice as hyperlactatemia and lactic acidosis occur frequently in veterinary ICU patients (shock, low cardiac output, acute liver failure, sepsis, neoplasia, peritonitis, poisoning and drug therapy). The reference values for the lactate concentration in dogs at rest are low than 2.0 mmol L-1 to 3.5 mmol L-1. According to studies in humans, a single measurement of lactate concentration is associated with the prognosis of survival. In veterinary medicine, there seems to be a relationship between lactate concentration and outcome in dogs (Bernardin 1996, de Papp et al. 1999). In our study, the lactate concentration was 2.4±1.2 mmol L-1 without any statistically significant difference among the groups (p=0.368).
Limitations of the present study include all those factors that affect the Pdi measurement. First of all, the Pdi measurement with balloon catheters is feasible only under general anaesthesia in dogs and the insertion of the balloon catheter was impossible in some cases as it has been referred in the results. So, it cannot be applied to all animals in the ICU and in our study, the measurement of Pdi was not applicable in all cases.
Conclusion
In summary, the technique of Pdi measurement with utilization of balloon catheters can be successfully applied in dogs in the ICU. Furthermore, Pdi measurement can be a useful tool for the assessment of diaphragmatic function in critically ill dogs at the admission and/or during their hospitalization in the ICU.
Conflict of interest
The authors declare no conflicts of interest.
This research is co-financed by Greece and the European Union (European Social Fund- ESF) through the Operational Programme «Human Resources Development, Education and Lifelong Learning» in the context of the project “Reinforcement of Postdoctoral Researchers” (MIS- 5001552), implemented by the State Scholarships Foundation (ΙΚΥ).

References
- Aubier M, Tippenbach T, Roussos C (1981) Respiratory muscle fatigue during cardiogenic shock. J Appl Physiol.
- Benditt JO (2005) Esophageal and gastric pressure measurements. Respir Care 50, 68–75.
- Berger D, Bloechlinger S, von Haehling S et al. (2016) Dysfunction of respiratory muscles in critically ill patients on the intensive care unit. J Cachexia Sarcopenia Muscle 7, 403–412.
- Bernardin G (1996) Blood pressure and arterial lactate level are early indicators of short-term survival in human septic shock. Intensive Care Med 22, 17–25.
- Bolton CF, Gilbert JJ, Hahn AF et al. (1984) Polyneuropathy in critically ill patients. J Neurol Neurosurg Psychiatry 47, 1223–1231.
- Calabro JM, Prittie JE, Palma DAD (2013) Preliminary evaluation of the utility of comparing SpO2/FiO2and PaO2/FiO2 ratios in dogs. J Vet Emerg Crit Care 23, 280–285.
- Callahan LA, Supinski GS (2009) Sepsis-induced myopathy. Crit Care Med 37, S354–S367.
- Canet J, Gallart L (2013) Predicting postoperative pulmonary complications in the general population. Curr Opin Anaesthesiol 26, 107–115.
- Demoule A, Jung B, Prodanovic H et al. (2013) Diaphragm dysfunction on admission to the intensive care unit: Prevalence, risk factors, and prognostic impact - A prospective study. Am J Respir Crit Care Med 188, 213–219.
- Demoule A, Molinari N, Jung B et al. (2016) Patterns of diaphragm function in critically ill patients receiving prolonged mechanical ventilation: a prospective longitudinal study. Ann Intensive Care 6, 75.
- Doorduin J, Van Hees HWH, Van Der Hoeven JG et al. (2013) Monitoring of the respiratory muscles in the critically Ill. Am J Respir Crit Care Med 187, 20–27.
- Esteban A, Anzueto A, Alía I et al. (2000) How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med 161, 1450–1458.
- Esteban A, Frutos F, Tobin MJ et al. (1995) A Comparison of Four Methods of Weaning Patients from Mechanical Ventilation. N Engl J Med 332, 345–350.
- Hayes G, Mathews K, Kruth S et al. (2010) Illness severity scores in veterinary medicine: What can we learn? J Vet Intern Med 24, 457–466.
- Hermans G, Agten A, Testelmans D et al. (2010) Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: A prospective observational study. Crit Care 14, R127.
- Heunks LMA, Doorduin J, Van Der Hoeven JG (2015) Monitoring and preventing diaphragm injury. Curr Opin Crit Care 21, 34–41.
- Hubmayr RD, Sprung J, Nelson S (1990) Determinants of transdiaphragmatic pressure in dogs. J Appl Physiol 69, 2050–2056.
- Hubmayr RD, Sprung J, Nelson SB (1990) Effect of lung volume and respiratory impedance on transdiaphragmatic pressure and muscle tension in dogs. CHEST J 97, 69S.
- Hussain SN, Roussos C, Magder S (1988) Autoregulation of diaphragmatic blood flow in dogs. J Appl Physiol 64, 329–336.
- Hussain SN, Simkus G, Roussos C (1985) Respiratory muscle fatigue: a cause of ventilatory failure in septic shock. J Appl Physiol 58, 2033–2040.
- Jaber S, Petrof BJ, Jung B et al. (2011) Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 183, 364–371.
- Jolley SE, Bunnell AE, Hough CL (2016) ICU-Acquired Weakness. Chest 150, 1129–1140.
- Kress JP, Hall JB (2014) ICU-Acquired Weakness and Recovery from Critical Illness. N Engl J Med 30, 1626–1635.
- Laghi F, Cattapan SE, Jubran A et al. (2003) Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med 167, 120–127.
- Matthay MA, Ware LB, Zimmerman GA (2012) The acute respiratory distress syndrome. J Clin Invest 122, 2731–2740.
- McFarlane I, Rosenthal F (1977) Severe myopathy after status asthmaticus. Lancet 2, 615.
- Ochala J, Renaud G, Diez ML et al. (2011) Diaphragm muscle weakness in an experimental porcine intensive care unit model. PLoS One 6, e20558.
- Olsen CW (1956) Lesions of peripheral nerves developing during coma. J Am Med Assoc 160, 39–41.
- Ora J, Laveneziana P, Wadell K et al. (2011) Effect of obesity on respiratory mechanics during rest and exercise in COPD. J Appl Physiol 111, 10–19.
- Osler W (1915) The principles and practice of medicine, designed for the use of practitioners and students of medicine D. Appleton, ed. J Nerv Ment Dis 21, 384.
- de Papp E, Drobatz KJ, Hughes D (1999) Plasma lactate concentration as a predictor of gastric necrosis and survival among dogs with gastric dilatation-volvulus: 102 cases (1995-1998). J Am Vet Med Assoc 15, 49–52.
- Pavlidou K, Savvas I, Moens Y et al. (2014) A minimally invasive method for clinical trans-diaphragmatic pressure measurement in anaesthetized dogs. Vet Anaesth Analg 41, 278–283.
- Pavlidou K, Savvas I, Moens YPS et al. (2013) The Effect of Four Anaesthetic Protocols for Maintenance of Anaesthesia on Trans- Diaphragmatic Pressure in Dogs. PLoS One 8, e75341.
- Rubenfeld GD, Caldwell E, Peabody E et al. (2005) Incidence and Outcomes of Acute Lung Injury. N Engl J Med 353, 1685–1693.
- Waterman AE, Hashim MA (1991) Measurement of the length and position of the lower oesophageal sphincter by correlation of external measurements and radiographic estimations in dogs. Vet Rec 129, 261-264
- Watson AC, Hughes PD, Harris ML et al. (2001) Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med 29, 1325–1331
Corresponding author:
Kiriaki Pavlidou
e-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.



