Eleni Prastiti DVM, Zoi Tzenetidou DVM, Christos Κoutinas DVM, PhD, George Kazakos DVM, PhD
Companion Animal Clinic, School of Veterinary Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
MeSH keywords:
cardiac arrhythmias, cat, dog
Abstract
Disorders of the heart rate are frequently observed in the perioperative period. Companion animals with cardiac or non-cardiac disorders, as well as healthy animals undergoing surgery may develop cardiac arrhythmias during the perianaesthetic period. Moreover, in specific surgical disorders affecting organs such as the spleen, the lower urinary tract and the canine gastric dilatation-volvulus syndrome, perioperative cardiac arrhythmias may develop. Identification of the type of arrhythmia and recognition of the cause are based on the continuous monitoring, visual examination of the mucous membranes, peripheral pulse palpation, assessment of temperature, electrocardiography (ECG), capnography, pulse oximetry, and blood pressure measurement. Findings resulting from arrhythmias, including hypotension, dyspnoea, mucosal congestion, hypothermia, decreased level of consciousness, decreased urine output, and increased levels of lactic acid, are indicators of haemodynamic instability and necessitate immediate management of the underlying disorder and often simultaneous symptomatic treatment of the emerging arrhythmia. Moreover, in cases when clinical signs have not yet manifested but certain “critical” characteristics of arrhythmias are observed on the ECG, it is recommended to administer appropriate antiarrhythmic drugs in order to prevent the manifestation of clinical signs or even sudden death. The type (tachyarrhythmia, bradyarrhythmia) as well as the origin (supraventricular, ventricular) of the arrhythmia determine the selection of antiarrhythmics that are appropriate for each case.
Introduction
Cardiac arrhythmias or dysrhythmias are disorders of the heart rate, which include changes in the heart rate, premature complexes and conduction system disorders (Martin 2007).
Perioperative cardiac arrhythmias are fairly common. Companion animals with primary cardiac disorders such as degenerative mitral valve disease, as well as animals with non-cardiac conditions that necessitate surgical management, or healthy animals that undergo routine surgery (e.g. ovariohysterectomy), are likely to develop perioperative arrhythmias (Oyama 2015). It is debated in several studies whether there is a correlation between perioperative arrhythmias and increased morbidity and mortality rates (Beck 2006, Mackenzie et al. 2010, Wendelburg et al. 2014).
In all cases the clinician needs to be aware of the physiological alterations in the heart rate of the anaesthetised patient, in order to differentiate them from what is abnormal and to intervene with the appropriate measures for the prevention or management of the latter, if necessary.
Mechanisms of arrhythmia formation
Arrhythmias are differentiated according to their origin, in disorders of impulse formation (automaticity) and/or disorders of conduction. Disorders of impulse formation can produce bradyarrhythmias, as well as tachyarrhythmias, whereas disorders in the cardiac conduction system can lead to delay or even block of the impulse conduction. Furthermore, arrhythmias can occur from ectopy (early or ectopic depolarisation) (Schober 2010).
Τhe specific cause of a perioperative arrhythmia cannot be easily identified in all cases, because arrhythmogenesis can arise from various conditions, such as inflammation, sepsis, hypoxia, oxidative stress, ischaemia and myocardial fibrosis (Oyama 2015).
The most common arrhythmias based on their mechanism of formation are shown in Table 1.
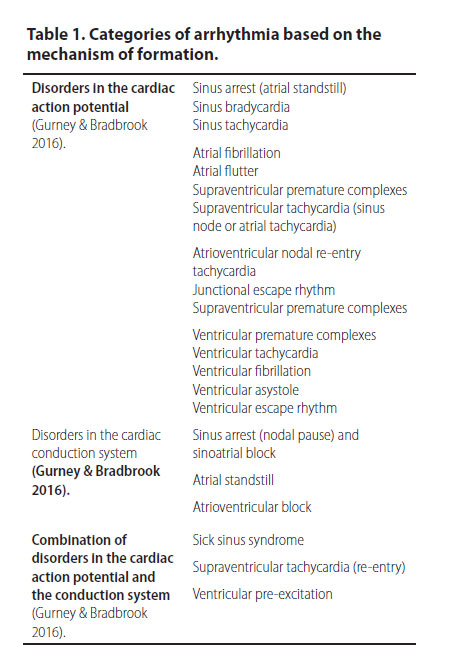
Cardiac disorders
The anaesthetic management of a case with congenital or acquired cardiac disorders, and/or chronic congestive heart failure is challenging and requires knowledge, experience and adequate preparation. The type of observed cardiac arrhythmias and their aetiopathogenesis depend on the underlying cardiac disease. Stabilisation should be the first priority before any surgical procedure, whereas prior to induction of anaesthesia an electrocardiogram (ECG) is recommended among other diagnostic tools, in order to differentiate arrhythmias caused by the drugs used in the anaesthetic protocol. Each case should be closely monitored with an ECG until full recovery (Gurney & Bradbrook 2016).
Even though primary cardiac disease is one of the main risk factors for perianaesthetic arrhythmias, the aim of this paper is to focus on the diagnosis and management of arrhythmias in cases with non-cardiac disease, considering that the latter pose a greater diagnostic challenge, since the occurrence of arrhythmias in such cases is often unexpected and they are therefore underdiagnosed and undermanaged.
Perianaesthetic monitoring
During the perianaesthetic period the identification of arrhythmias and the decision to administer antiarrhythmic drugs is based on the continuous monitoring (Oyama 2015). The parameters that should be constantly monitored include the following:
- Electrocardiography: It provides not only information relevant to the exact type of arrhythmia and the architecture of the heart, but also it usually assists in revealing the underlying cause (Cornick-Seahorn 2006).
- Heart rate: Intraoperative use of an oesophageal stethoscope is necessary if ECG is not available (Cornick-Seahorn 2006). Heart rates over 180 min-1 or under 50 min-1 in dogs and over 220 min-1 or under 90 min-1in cats, are related to increased risk for development of clinical signs (Oyama 2015).
- Pulse quality: Except of the estimation of the heart rate and the evaluation of the cardiac rhythm, the femoral artery or other superficial arteries are palpated to diagnose pulse deficits and evaluate pulse quality, as that arrhythmias tend to change the latter depending on the type and severity of the arrhythmia (Bonagura 2008).
- Capillary refill time (CRT) and mucous membrane colour: Normally, the mucous membrane colour is pink, and CRT is less than 2 sec. A bright red coloration with a simultaneous decrease of CRT may be caused by vasodilation resulting from hypercapnia, sepsis, hypothermia and anaesthetic drug administration (propofol, inhalational anaesthetics), which are risk factors for the development of arrhythmias (Congdon 2015). Mucosal pallor with simultaneous increase in CRT indicates hypotension, shock, anaemia, hypothermia or it can result from drugs such as α-2 adrenergic receptor agonists (peripheral vasoconstriction) and some opioids.
- Temperature measurement: Hypothermia can be a risk factor for arrhythmias as well as a clinical sign caused by an arrhythmia due to the resulting haemodynamic instability (Haskins 2007, Oyama 2015).
- Respiratory rate and dyspnoea: The thoracic wall and/or the anaesthetic machine reservoir bag is observed in order to measure the respiratory rate, to estimate respiration depth and to diagnose dyspnoea. Animals with arrhythmias should receive oxygen throughout the perioperative period. Tachypnoea in the presence of arrhythmia may suggest hypoxia, hypercapnia and acidosis (Macintire 2006).
- Arterial blood pressure: Arterial pressure can provide information regarding the cardiac output, the condition of peripheral blood vessels, and therefore tissue perfusion. Hypotension in the perianaesthetic period may be caused by anaesthetic drugs (e.g. phenothiazines, isoflurane), arrhythmias or antiarrhythmic drugs administered as treatment for tachyarrhythmias (Mazzaferro 2001, Macintire 2006, Oyama 2015). In order to maintain adequate perfusion in the brain and kidneys, the minimum mean arterial pressure required is 60 mmHg in both dogs and cats (Duke-Novakovski & Carr 2015).
- Pulse oximetry: For the identification of hypoxaemia, oxyhaemoglobin saturation should be measured in arterial blood (SpO2). In case of arrhythmia, hypoxaemia can be the underlying cause for the development of arrhythmia (myocardial ischaemia), as well as the result of an arrhythmia (haemodynamic destabilisation). Companion animals breathing atmospheric air should have an SpO2 above 92%, whereas animals receiving 100% Ο2 should have SpO2 above 98%. It is worthy of note that macroscopically visible cyanosis of the mucosae occurs when SpO2 is less than 74%, and there is no moderate or severe anaemia (Ko et al. 2001, Epstein 2005, Pypendop 2005).
- Capnometry-capnography: In order to have a normal capnogram, normal cellular metabolism, normal perfusion and normal alveolar ventilation are required. Any observed alterations on the capnogram can often be an indication of haemodynamic instability, among other causes (Creedon 2013).
- Urine output: In the absence of renal disease, urine output is a sufficient indicator of renal perfusion and cardiac output. Normally, urine output exceeds 1 ml kg-1 h-1 and it should be measured often as an indicator of adequate perfusion of the renal tissue (Creedon 2013).
- Laboratory testing: These include arterial blood gas analysis as well as electrolyte, lactic acid and cardiac troponin-I estimation. Acid- base and electrolyte disorders can be an underlying cause for temporary arrhythmias as well as a result of haemodynamic destabilisation resulting from the arrhythmias (Cohen & Tilley 1979). Lactic acid and troponin-I levels provide information about tissue perfusion and possible myocardial tissue damage, respectively (Creedon 2013, Hamacher 2015).
Benign arrhythmias
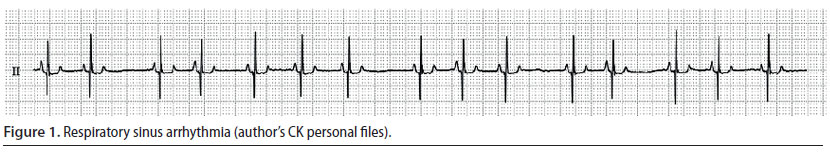
Respiratory sinus arrhythmia (Figure 1)
Respiratory sinus arrhythmia is a sinus rhythm in which the heart rate increases and decreases regularly (Martin 2007). It usually depends on respiration, i.e. the heart rate increases on inspiration and decreases on expiration. Moreover, it indicates an increased vagal tone because it is related to the increased effect of vagal nerve stimulation on the sinus node and when present, it is an indicator of myocardial health, as long as sympathetic tone stimulation is usually related to heart failure (Martin 2007). Respiratory sinus arrhythmia may be observed in healthy dogs at rest, after the administration of α-2 adrenergic agonists and in brachycephalic dog breeds (Gross 2009, Papich 2016). Other than these cases in which respiratory sinus arrhythmia is considered to be “benign”, it can also emerge in sick dogs with respiratory, neurological, or ophthalmological disorders as well as in cases in which the stomach or intestine are affected. In contrast with dogs, respiratory sinus arrhythmia is rare in cats and it can be noted in the disorders that were previously mentioned, as well as during dyspnoea or in cats with cardiomyopathies. Respiratory sinus arrhythmia, in and of itself, does not require any treatment (Gompf 2011).
Normal variations in the heart rate
In healthy animals and humans, the R-R intervals of the ECG are not regular and may vary according to the normal effects of the changes in the balance of the autonomic nervous system (Spyer 1994). Τhis variation in the heart rate can provide valuable information about the state of myocardium. In particular, patients with congestive heart failure may have increased heart rate and reduced variation of cardiac rhythm due to chronic stimulation of the sympathetic nervous system (Nolan et al. 1992). Therefore, if extensive variations in the heart rate are noted, this is indicative either of healthy myocardial tissue, or an underlying cardiomyopathy in which congestive heart failure has not yet been established (Häggström et al. 1996). It should be noted that the exact estimation of this parameter requires specialised software.
Identification and treatment of arrhythmias
Tachyarrhythmias
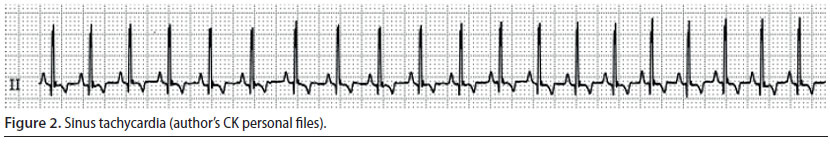
Sinus tachycardia (Figure 2)
- This is a sinus rhythm however the heart rate is higher than normal (more than 140 min-1) (Oyama 2015).
- Every cardiac contraction is followed by a palpable pulse, however when the heart rate is too high, the pulse may become weak (Oyama 2015).
Sinus tachycardia is not a dangerous/ life-threatening arrhythmia. It usually occurs after the administration of ketamine, atropine, and thiobarbiturates or after intense stimulation of the sympathetic nervous system such as in cases of perioperative pain, anaemia, hypovolemia, and insufficient depth of general anaesthesia (Martin 2007, William 2007). Management is based on addressing the underlying cause, meaning treatment is based on analgesics, appropriate intravenous fluids and adjusting the depth of general anaesthesia (Cohen 1979, Stepien 2005).
Atrial fibrillation (Figure 3)
- The wave of depolarisation begins at random time points, originating from an atrial site, without leading to adequate atrial contraction (Martin 2007).
- During auscultation the cardiac rhythm is chaotic, whereas during palpation of the pulse there are pulse deficits in 50% of the number of cardiac contractions (Martin 2007).
- During ECG evaluation, there are no identifiable P waves, QRS complexes are normal, whereas the R-R intervals are chaotically irregular (Martin 2007).
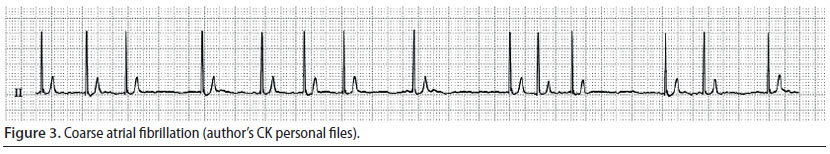
Atrial fibrillation is the most commonly diagnosed arrhythmia in companion animals, it is usually caused by underlying heart disease, and results in reduction of cardiac output (Martin 2007). Consequently, when it is noted during the perianaesthetic period, it may be assumed that it was present prior to this time (Bright 2012). When this arrhythmia is observed, the authors usually discontinue the anaesthetic process, even earlier than normal (as long as the surgery stage can allow it), and cardiological evaluation is performed, in order to determine if this arrhythmia was temporary. However, atrial fibrillation has been noted in cases without morphological abnormalities of the heart, during surgical manipulations that increase vagal tone (e.g. endotracheal intubation) and after opioids had been previously infused (butorphanol, fentanyl, morphine) (Moïse et al. 2005). An intravenous bolus of lidocaine has been successfully used to treat acutely emerging atrial fibrillation caused by parasympathetic stimulation (Moïse et al. 2005).
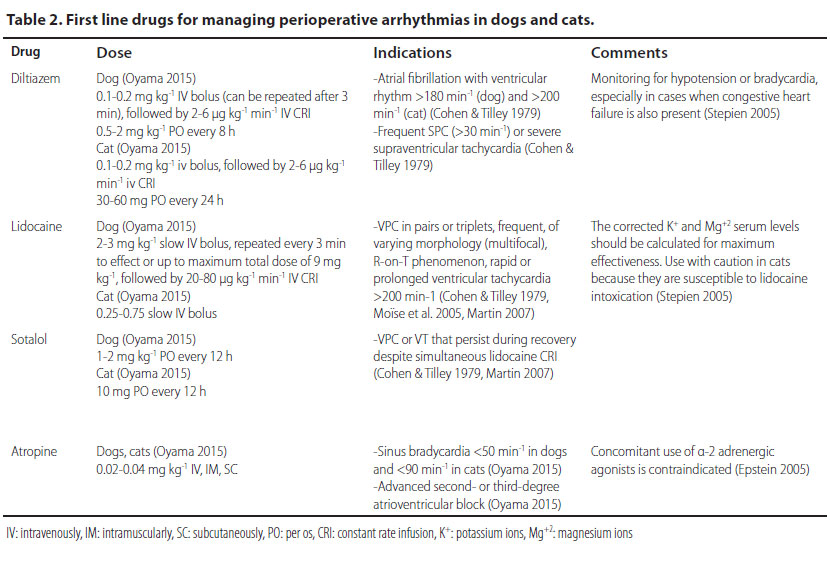
Τhe primary treatment goal after diagnosing atrial fibrillation is to restore the heart rate. Heart rates of more than 180 min-1 (dog) or 200 min-1 (cat) require slowing of the heart rate, which is accomplished by the intravenous infusion of calcium channel blockers (e.g. diltiazem) (Stepien 2005). In particular, an intravenous bolus infusion is recommended followed by repeated intravenous infusions or constant rate infusion (CRI), so that the cardiac rhythm can be maintained within the acceptable range for a case of atrial fibrillation, i.e. in the range of 140-160 min-1 (dog) or 160-180 min-1 (cat) (Stepien 2005, Bright 2012).
Supraventricular premature complexes (Figure 4) and supraventricular tachycardia
- Supraventricular premature complexes (SPC) originate from an extranodal site or sites located above the ventricles, which depolarise normally. A group of three or more SPC is categorised as supraventricular tachycardia (ST) (Martin 2007).
- Every premature beat results in weak or absent pulse. Therefore, in ST the heart rate is higher than the number of palpable pulses (Martin 2007).
- During ECG evaluation, QRS complexes occur prematurely, however they appear normal. Furthermore, P waves may or may not be observed, however when they are identifiable, they are usually abnormal in appearance. The P-R interval is shorter than the same interval during sinus rhythm.
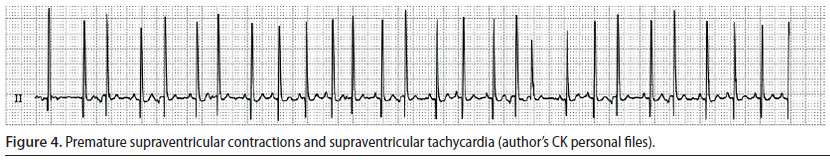
When SPC are single and occur infrequently, treatment is not required, because they do not lead to reduced cardiac output. In such cases, it is adequate to remove the underlying cause in order for the arrhythmia to subside (Stepien 2005, Martin 2007). In contrast, when SPC are frequent or when there is severe ST -which can often reach 400 min-1 (dog), this can result in severely reduced cardiac output (Stepien 2005) In such cases, treatment should be directed to the underlying cause, as well as aim to reduce the frequency of SPC or to restore sinus rhythm and accomplish a normal heart rate, respectively (Martin 2007).
The intravenous infusion of diltiazem (Table 2) is the treatment of choice, because it can induce a rapid reduction in ventricular response to supraventricular tachycardia, therefore it reduces the heart rate (Bright 2012). Furthermore, the negative inotropic effect of diltiazem is less potent than other antiarrhythmic drugs used in treating ST, an important advantage for animals under general anaesthesia, in which the administered anaesthetic drugs usually have a negative inotropic effect on the myocardium (Schwartz 2009).
In conscious animals, a combination of drugs and vagal manoeuvres is recommended, such as mild pressure on the eyeballs or carotid sinus massage, behind the angles of the lower mandible. These manoeuvres may stop or slow ST, assisting in its identification (Stepien 2005, Martin 2007). However, the restoration of sinus rhythm may be brief, difficult or impossible, in cases in which only vagal manoeuvres are performed without simultaneous administration of antiarrhythmic drugs (Stepien 2005).
Ventricular premature complexes (Figure 5) and ventricular tachycardia (Figure 6)
- Ventricular premature complexes (VPC) originate from one or more extranodal sites located in the ventricles. The impulses are then conducted from one myocardial cell to another, bypassing the specialised conduction system in an abnormal direction. A group of three or more VPCs is classified as ventricular tachycardia (VT) (Martin 2007).
- During ECG evaluation, QRS complexes have an abnormal and wide morphology and their duration is usually 50% longer than normal. Τhe Τ waves are usually larger than normal and in opposite direction to the QRS complexes of the VPC, whereas there is a characteristic absence of previous P waves. When a VPC appears prematurely the «R-on-T» phenomenon may be observed, i.e. the VPC falls on the T wave of the previous contraction, because the depolarisation of the ventricles begins before repolarisation has been completed (Martin 2007). The presence of QRS complexes of varying morphology is also of clinical importance, because it is an indicator of the existence of more than one ectopic pacemaker (multifocal) (Martin 2007, Bright 2012). Τhe appearance of «R-on-T» phenomenon, as well as QRS complexes of various morphology are exceptionally important findings, because they can lead to sudden death and for that reason antiarrhythmic treatment should be provided immediately (Martin 2007, Bright 2012)
- The severity of VPCs is evaluated according to their effect on cardiac output. When they are single and rare, there is no reduction in cardiac output, therefore the identification and the removal of the underlying cause of the arrhythmia are adequate management measures. However, when VT is severe enough to cause a reduction in cardiac output or when the «R-on-T» phenomenon is observed, it is considered necessary to administer antiarrhythmic drugs (Stepien 2005).
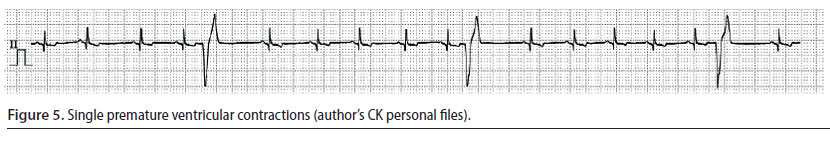
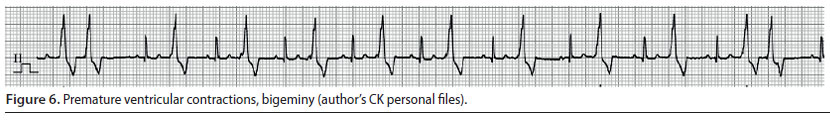
The intravenous infusion of lidocaine (Table 2) is the cornerstone of the treatment and the drug of choice for managing acutely emerging VT. If bolus infusion of lidocaine is effective, then it is important that it is immediately followed by lidocaine in intravenous CRI. It should be noted that until lidocaine has reached the desired serum levels, it is mandatory to repeat bolus infusions. If the arrhythmia is still present during recovery, antiarrhythmic drugs per os (e.g. sotalol) can be added to the treatment regimen. Antiarrhythmic drug administration per os can begin while CRI is still administered. In such cases, the CRI should be reduced by 50% every 6 hours (Stepien 2005, Martin 2007).
Cats may develop clinical signs of lidocaine toxicity, most commonly from the cardiovascular system (hypotension, bradycardia, arrhythmias, asystole), the gastrointestinal tract (salivation, vomiting), and the central nervous system (ataxia, nystagmus, muscle tremors, seizures). For that reason, the use of lidocaine must be absolutely justified, and the dose rate should be strictly followed (Martin 2007).
After antiarrhythmic drugs have been administered in order to manage tachyarrhythmias, thorough control of the mean arterial pressure and heart rate are recommended. Due to the negative inotropic and chronotropic effect of such drugs, the risk of hypotension or bradycardia is increased, especially in cases of congestive heart failure (Bright 2012).
Bradyarrhythmias
Perioperative bradyarrhythmias are usually temporary and rarely result in clinical signs that requiring medical intervention (Bright 2012).
Sinus bradycardia
-
The rhythm is sinus, but the heart rate is slower than normal (less than 60 min-1 in dogs and less than 90 min-1 in cats) (Martin 2007).
-
Every contraction is followed by a palpable pulse (Martin 2007).
-
During ECG evaluation the P-QRS complexes are normal but with a slower frequency (Rudloff & Raffe 2012).
Sinus bradycardia is usually the effect of anaesthetic drugs such as α-2 adrenergic agonists and opioids, and it subsides after the expiration of their action or the administration of antagonists or atropine. In cases when sinus bradycardia is caused by electrolyte disorders, increased vagal tone, and severe hypovolemia, managing the these disorders is sufficient in order to restore the heart rate (Stepien 2005). In cases of persistent or life-threatening bradycardia, as in lessa than 50 min-1 in dogs and less than 90 min-1 in cats, or when clinical signs of reduced cardiac output are observed, the use of atropine intravenously is justified (Table 2). At this point it is worthy of note that the administration of atropine in order to manage bradyarrhythmia caused by α-2 adrenergic agonists is contraindicated, because myocardial workload and oxygen demand are increased and severe hypertension may develop (mean arterial blood pressure up to 210 mmHg) (Ko 2001). α-2 Adrenergic agonists are usually selected for healthy patients, because they can induce bradycardia and consequent reduction in cardiac output. Such bradycardia usually does not necessitate treatment. However, if it is severe, the authors recommend the partial or total antagonism of the α-2 adrenergic agonist with atipamezole at the same time as other supportive measures are usually provided (fluids, oxygen). In cases of partial antagonism, anaesthesia is maintained.
Atrioventricular block
This is a failure to conduct the impulse wave through the atrioventricular node and it can be classified as partial (first- or second-degree) or complete (third-degree) block (Martin 2007).
1) First-degree atrioventricular block
- This is noted when there is a delay in conduction through the atrioventricular node and a sinus rhythm is usually present (Martin 2007).
- The heart rate and rhythm are within normal range and therefore no abnormalities are noted during cardiac auscultation and pulse palpation (Martin 2007, Rudloff & Raffe 2012).
- During ECG evaluation, the P-QRS complexes are normal, but the P-R intervals are wider than normal (Brockman et al. 1995).
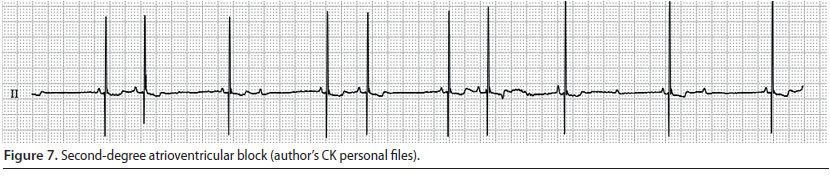
Second-degree atrioventricular block (Figure 7)
- This is observed when the impulse wave conduction through the atrioventricular node is intermittent, i.e. there are atrial depolarisations which are not followed by the corresponding ventricular depolarisations (Martin 2007).
- During cardiac auscultation, atrial contraction can be perceived as a faint sound during atrial depolarisation (Martin 2007).
- During the ECG evaluation, P waves that are not always followed by QRS complexes are observed. Usually there are two P waves for every QRS complex, but in sustained or severe atrioventricular block, there are more P waves for every normal P-QRS complex (Tilley & Smith 2016).
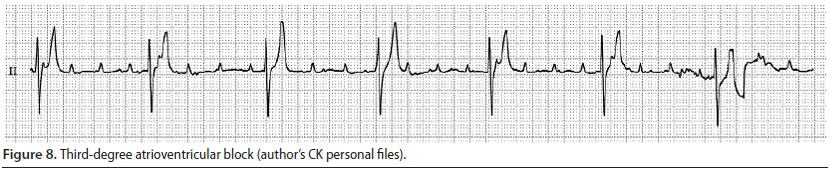
3) Third-degree atrioventricular block (Figure 8)
- There is a complete failure of conduction of the impulse waves through the atrio-ventricular node, therefore the atria and the ventricles constrict independently. The atrial rate is faster than the ventricular. Control of the ventricles is taken over by an ectopic pacemaker which is located under the block area, in the lower half of the sinus node, the bundle branches, or the Purkinje fibres. In the first case, QRS morphology will be normal (junctional escape beats) with a heart rate of 60-70 min-1, whereas in the second, QRS-T morphology will be abnormal (ventricular escape beats) with a heart rate of 30-40 min-1 (idioventricular rhythm) (Martin 2007, Tilley & Smith 2016).
- During auscultation, severe bradycardia is noted with good pulse quality and atrial contraction is perceived as a barely auscultated sound of higher frequency unrelated to the normal S1 and S2 sounds of contraction and dilation of the ventricles (Martin 2007).
- During ECG evaluation, there is dissociation between P waves and QRS complexes, and P waves appear more frequently than QRS complexes (Martin 2007).
The first- and second-degree atrioventricular block in the perianaesthetic period is related to the administration of opioids and α-2 adrenergic agonists (xylazine, dexmedetomidine) (William 2007). In such cases treatment is not necessary, as long as the mean arterial blood pressure is maintained over 60 mmHg (Smith 2002). Medical treatment is provided in cases with clinical signs of reduced cardiac output, in second-degree atrioventricular block with heart rate below 40 min-1 in dogs and below 60 min-1 in cats, and in third-degree atrioventricular block. Treatment is mainly based on the intravenous infusion of atropine (Table 2) (Martin 2007).
Criteria for therapeutic intervention
Haemodynamic destabilisation caused by the presence of cardiac arrhythmias necessitates the administration of appropriate antiarrhythmic drugs concurrently with cardiovascular support measures. Furthermore, in cases in which clinical signs are not present yet, but there are certain “critical” features of arrhythmias on the ECG, the administration of antiarrhythmic drugs is still recommended, in order to prevent the development of clinical signs or eventually sudden death of the patient. Clinical signs of haemodynamic destabilisation (Schwartz 2009, Oyama 2015):
- Hypotension
- Dyspnoea
- Congested or pale mucosae
- Insufficient tissue perfusion (altered level of consciousness, reduced urine output, increased levels of lactic acid)
- Hypothermia
Critical signs on the ECG:
- Duration of arrhythmia longer than 30 sec (Oyama 2015).
- Atrial fibrillation with a ventricular rate of over 180 min-1 (dog) or over 200 min-1 (cat) Stepien 2005).
- Persistent heart rate over 180 min-1 or below 50 min-1 in dogs and over 220 min-1 or below 90 min-1 in cats (Oyama 2015).
- Frequent SPCs (over 30 min-1) or severe ST (Stepien 2005).
- VPCs in pairs or triplets, frequent, of variable morphology, R-on-T phenomenon, rapid or prolonged ventricular tachycardia over 200 min-1(Stepien 2005, Martin 2007, Schwartz 2009, Oyama 2015).
- Third-degree atrioventricular block (Martin 2007).
First-line drugs for managing perioperative arrhythmias and the exact doses of administration are summarised in Table 2.
Disorders associated with perioperative arrhythmias
As previously mentioned, perioperative arrhythmias result from temporary and reversible pathological conditions that usually develop during the perioperative period. The severity of such temporary arrhythmias is variable, and it may include a wide spectrum of arrhythmias, from “innocent” sinus bradycardia to life-threatening ventricular tachycardia. Usually, the management of the predisposing factor in the initial stages is adequate for the treatment of the arrhythmias (Oyama 2015).
In Table 3 are summarised: 1) examples of pathological disorders which occur as perianaesthetic complications in which temporary arrhythmias are anticipated, 2) the aetiopathogenesis of arrhythmias, and 3) the type of arrhythmias.
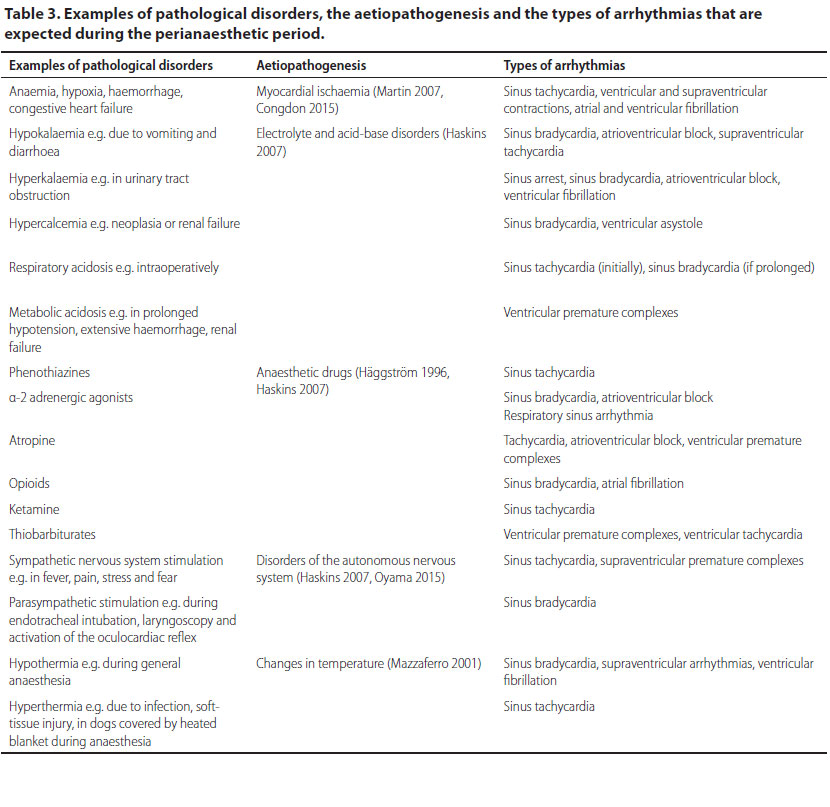
Some surgical disorders affecting organs such as the spleen, lower urinary tract and cases of canine gastric dilatation-volvulus syndrome, have been consistently implicated in the development of perioperative cardiac arrhythmias and clinicians should be adequately prepared to manage them prior to, during and/or after surgery.
Gastric dilatation with or without volvulus
In cases with gastric dilatation with or without volvulus, ventricular arrhythmias are observed in 40-50% of cases in the perianaesthetic period (Brockman et al. 1995, Roux 2012). In particular, the development of arrhythmias is anticipated within 12-72 hours from the time that clinical signs are evident, necessitating the constant monitoring of such cases with ECG for the duration of hospitalisation (Roux 2012). Some of the causes of arrhythmias are at the same time deteriorating factors and these include myocardial ischemia, acid-base and electrolyte abnormalities, increased tone of the sympathetic nervous system, ischaemia-reperfusion injury and release of the myocardial depressant factor from the pancreas (Roux 2012). Even though it has been shown that the development of cardiac arrhythmias during splenectomy, gastrectomy or gastric wall necrosis is a negative prognostic factor for such cases, other researchers claim that the presence of arrhythmias does not affect the mortality rate (Brockman et al. 1995, Brourman et al. 1996). In cases of life-threatening arrhythmias and resulting haemodynamic destabilisation, the appropriate antiarrhythmic drugs are administered at the same time as oxygen, after acid/base and electrolyte disorders have been managed (Roux 2012). An interesting study presents cases with gastric dilatation-volvulus that received lidocaine intravenously as a prophylactic measure prior to gastric decompression and intravenously administration of fluids, followed by lidocaine CRI for the first 24 hours. The pre-emptive administration of lidocaine in these cases significantly reduced the incidence of perioperative arrhythmia, acute renal injury and the postoperative hospitalisation time (Bruchim et al. 2012).
Splenectomy
Animals undergoing splenectomy due to neoplasia, splenic torsion or immune-mediated haematological disorders (e.g. immune-mediated thrombocytopenia, immune-mediated haemolytic anaemia), are frequently present with ventricular arrhythmias (Keyes et al. 1993). In such cases, arrhythmias are caused from the release of emboli or oxygen free radicals in the systemic circulation during surgical manipulations of the spleen, arterial hypotension, anaemia and the effect of other suppressive factors on myocardial tissue (Keyes et al. 1993, Marino et al. 1994). Even though arrhythmias may emerge during the perioperative period, they develop more often intra-operatively and/or 5-12 h post-operatively, procedure and therefore it is recommended to continuously monitor these patients with ECG throughout of hospitalisation Keyes et al. 1993, Marino et al. 1994). The types of arrhythmia observed in cases of splenectomy rarely result in haemodynamic destabilisation and they have not been associated with increased mortality rates. The arrhythmias usually resolve within 2-5 days after the surgery, with the administration of oxygen and/or blood transfusion and the management of hypotension and metabolic disorders. However, in cases of severe arrhythmias leading to haemodynamic destabilisation, it is recommended to administer appropriate antiarrhythmic drugs (Moïse et al. 2005).
Sepsis
Diseases predisposing to sepsis such as pyometra, intestinal rupture, abscesses, etc. are very common in the clinical veterinary setting (Laforcade 2010). Regardless of aetiology, sepsis can result in sinus or ventricular tachycardia, premature ventricular complexes and reduced cardiac contractility, which in combination with insufficient circulating blood volume prior to stabilisation, and therefore reduced preload, can result in reduced cardiac output (Silverstein 2006, Schwartz 2009). In cases of sepsis, the aim of treatment is the prevention of septic shock and the management includes the administration of oxygen and intravenous fluids, the correction of metabolic and electrolyte disorders and the use of effective analgesia (Laforcade 2010). In cases with premature ventricular complexes and prolonged ventricular tachyarrhythmia, appropriate antiarrhythmic drugs are administered (Table 2) (Silverstein 2006, Schwartz 2009). Cases of septic shock often require the administration of vasopressors (dopamine) and/or positive inotropes (dobutamine) (Silverstein 2006, Laforcade 2010).
Hyperkalaemia
The ratio of intracellular to extracellular potassium concentration can affect muscle fibre conduction (skeletal and cardiac muscle). Therefore, in hyperkalaemia it is possible to observe bradycardia or sinus arrest due to extensive depolarisation and repolarisation of the cardiac conduction system. Perioperative hyperkalaemia has been observed in cases of reduced urine output (urinary tract obstruction, oliguric renal failure, urinary tract rupture), but also in cases of diabetes mellitus and extensive soft tissue trauma (Riordan & Schaer 2009). Bradycardia has been a consistent feature of the typical ECG in these patients, as well as small or absent P waves and an increase in T wave amplitude. In some cases, the emerging dysrhythmia can include sinus tachycardia, ventricular tachycardia and second-degree atrioventricular block (Tag & Day 2008). Electrocardiographic findings are not directly correlated to serum potassium levels, as they can be more directly affected by the trend of increasing or decreasing of potassium levels. Treatment of the emerging arrhythmia should aim in reducing hyperkalaemia, it can vary according to serum potassium levels and it is based on the redistribution of potassium from the extracellular to the intracellular space (Riordan & Schaer 2009). It is worthy of note that the management of hyperkalaemia should precede the administration of general anaesthesia.
Hyperthyroidism
Studies have proven that 60% of cats with untreated hyperthyroidism developed frequently severe tachycardia (over 240 min-1). In another study, 10% of cats undergoing thyroidectomy presented with ventricular and atrial dysrhythmias during surgery (Tag & Day 2008). Electrocardiographic changes caused by thyrotoxicity are due to the increased stimulation of the sympathetic nervous system and the stimulatory effect of thyroid hormones on myocardial tissue (Birchard et al. 1984). In cases of cats with hyperthyroidism needing to undergo surgery, it is necessary to induce a euthyroid state and normalise the heart rate before surgery, in order to reduce the risk of perianaesthetic arrhythmias (Peterson et al. 1992, Bond et al. 1988). Stimulation of the sympathetic nervous system during surgery can be prevented with sufficient analgesia and the appropriate anaesthetic protocol (Casamian 2009).
Conclusions
Disorders of the heart rate during the perianaesthetic period should be anticipated not only in animals with cardiac disease but also in cases undergoing certain surgical procedures which pose a higher risk for the development of arrhythmias, as well as in cases with non-cardiac disorders which undergo surgery. Continuous monitoring is the cornerstone for the identification of critical signs on the ECG and clinical signs of haemodynamic destabilisation, which necessitate the administration of the appropriate supportive and antiarrhythmic medical treatment.
Conflict of interest
The authors declare no conflicts of interest.
References
- Beck SP (2006) Risk factors associated with short-term outcome and development of perioperative complications in dogs undergoing surgery because of gastric dilatation-volvulus: 166 cases (1992- 2003). J Am Vet Med Assoc 229, 1934-1939.
- Birchard SJ, Peterson ME, Jacobson A (1984) Surgical treatment of feline hyperthyroidism: results of 85 cases. J Am Anim Hosp Assoc 20, 705-709.
- Bonagura JD (2008) Cardiopulmonary System. In: S. J. Βirchard, R. G. Sherding, eds. Saunders manual of small animal practice. 3rd edn. Saunders Elsevier, St Louis, pp. 1421-1731.
- Bond BR, Fox PR, Peterson ME, Skavaril RV (1988) Echocardiographic findings in 103 cats with hyperthyroidism. J Am Vet Med Assoc 192, 1546-1549.
- Bright JM (2012) Perioperative Cardiac Arrhythmias. In: M. J. Bojrad, E. Monnet. Mechanisms of Disease in Small Animal Surgery. 3rd edn. Teton NewMedia, Wyoming, pp. 1-11.
- Brockman DJ, Washabau RJ, Drobatz KJ (1995) Canine gastric dilatation/volvulus syndrome in a veterinary critical care unit: 295 cases (1986-1992). J Am Vet Med Assoc 207, 460-464.
- Brourman JD, Schertel ER, Allen DA, Birchard SJ (1996) Factors associated with perioperative mortality in dogs with surgically managed gastric dilatation-volvulus: 137 cases (1988-1993). J Am Vet Med Assoc 208, 1855-1858.
- Bruchim Υ, Itay S, Shira BH, Kelmer E, Sigal Y, Itamar A, Gilad S (2012) Evaluation of lidocaine treatment on frequency of cardiac arrhythmias, acute kidney injury, and hospitalization time in dogs with gastric dilatation volvulus. J Vet Emerg Crit Care 22, 419-427.
- Casamian D (2009) Cardiovascular effects of systemic or endocrine diseases. In: Proceeding of the SEVC Southern European Veterinary Conference & Congreso Nacional. Barcelona, Spain.
- Cohen RB, Tilley LP (1979) Cardiac Attythmias in the Anesthetized Patient. Vet Clin North Am Small Anim Pract 9, 155-167.
- Congdon JM (2015) Cardioavascular disease. In: L. B. C. Snyder, R. A. Johnson. Canine and Feline Anesthesia and Co-Existing Disease. Willey Blackwell, Wisconsin, pp. 1-54.
- Cornick-Seahorn J (2006) Monitoring critical care patients. In: Proceedings of the North American Veterinary Conference. Orlando, Florida, pp. 79-81.
- Creedon BJM (2013) Assessment and monitoring of blood volume and tissue perfusion. In: Proceedings of the Southern European Veterinary Conference and Congreso Nacional AVEPA. Barcelona, Spain.
- Duke-Novakovski T, Carr A (2015) Perioperative Blood Pressure Control and Management. Vet Clin North Am Small Anim Pract 45, 965-981.
- Epstein S (2017) Pulse oximetry. In: S. J. Ettinger, E. C. Feldman, E. Cote. Textbook of Veterinary Internal Medicine. 8th edn. Elsevier, Toronto, pp. 374-376.
- French A (2008) Arrhythmias: recognition and treatment. In: Proceedings of the 33rd World Small Animal Veterinary Congress. Dublin, Ireland, pp. 119-121.
- Gompf R (2011) Sinus arrhythmia. In: R. V. Morgan. Small animal practise client handouts. 2nd edn. Saunders Elsevier, Maryland Heights, p. 59.
- Gross DR (2009) Cardiovascular Effects of Anesthetics, Sedatives Postoperative Analgesic Agents, and Other Pharmaceuticals. In: D. R. Gross. Animal Models in Cardiovascular Research. 3rd edn. Springer-Verlag, New York, pp. 131-203.
- Gurney M, Bradbrook C (2016) Common ECG abnormalities in the perioperative period. In Practice 38, 219-28.
- Häggström J, Hamlin RL, Hansson K, Kvart C (1996) Heart rate variability in relation to severity of mitral regurgitation in Cavalier King Charles spaniels. J Small Anim Pract 37, 69-75.
- Hamacher L, Deorfelt R, Meuller M, Wess G (2015) Serum Cardiac Troponin I Concentrations in Dogs with Systemic Inflammatory Response Syndrome. J Vet Intern Med 29, 164-170.
- Haskins SC (2007) Monitoring Anesthetized Patients. In: V. W. Lumb, E. W. Jones. Veterinary anesthesia and analgesia. 4th edn. Blackwell Publishing, Oxford, pp. 533-560.
- Keyes ML, Rush JE, Couto CG, Autran de Morais HS (1993) Ventricular Arrhythmias in Dogs With Splenic Masses. J Vet Emerg Crit Care 3, 33-38.
- Ko JC, Fox SM, Mandsager RE (2001) Effects of preemptive atropine administration on incidence of medetomidine-induced bradycardia in dogs. J Am Vet Med Assoc 218, 52-58.
- Laforcade AM (2010) Management of septic peritonitis in dogs and cats. In: Proceedings of the 35th World Small Animal Veterinary Congress. Geneva, Switzerland.
- Macintire DK (2006) Monitoring Critically Ill Cats and Dogs. In: Proceeding of the north american veterinary conference. Orlando, Florida, pp. 263-265.
- Mackenzie G, Barnhart M, Kennedy S, DeHoff W, Schertel E (2010) A Retrospective Study of Factors Influencing Survival Following Surgery for Gastric Dilatation-Volvulus Syndrome in 306 Dogs. J Am Anim Hosp Assoc 46, 97-102.
- Marino DJ, Matthiesen DT, Fox PR, Lesser MB, Stamoulis ME (1994) Ventricular arrhythmias in dogs undergoing splenectomy: a prospective study. Vet Surg 23, 101-106.
- Martin M (2007) Small animal ECGs: Introductory Guide. 2nd edn. Blackwell Publishing, Oxford.
- Mazzaferro E, Wagner AE (2001) Hypotension During Anesthesia in Dogs and Cats: Recognition, Causes, and Treatment. Compendium 28, 728-737.
- Moïse NS, Pariaut R, Gelzer AM, Kraus MS, Jung SW (2005) Cardioversion with lidocaine of vagally associated atrial fibrillation in two dogs. J Vet Cardiol 7, 143-148.
- Nolan J, Flapan AD, Capewell S, MacDonald TM, Neilson JM, Ewing DJ (1992) Decreased cardiac parasympathetic activity in chronic heart failure and its relation to left ventricular function. Br Heart J 67, 482-485.
- Oyama M (2015) Perioperative Monitoring of Heart Rate and Rhythm. Vet Clin North Am Small Anim Pract 45, 953-963.
- Papich MG (2016) Medetomidine Hydrochloride. In: M. G. Papich. Saunders Handbook of Veterinary Drugs. 4th edn. Elsevier, St. Louis, pp. 481-483.
- Peterson ME, Keene B, Ferguson DC, Pipers FS (1982) Electrocardiographic findings in 45 cats with hyperthyroidism. J Am Vet Med Assoc 180, 934-937.
- Pypendop B (2005) Monitoring of the respiratory system. In: 50th Congresso Nazionale Multisala. Rimini, Italia.
- Riordan LL, Schaer M (2009) Potassium Disorders. In: D. C. Silverstein, K. Hopper. Small Animal Critical Medicine. 1st edn. Saunders Elsevier, Toronto, pp. 229-232.
- Roux (2012) How I treat...Gastric Dilation and Volvolus (GVD). In: Proceedings of the Southern European Veterinary Conference and Congreso Nacional de AVEPA. Barcelona, Spain.
- Rowland E, McKenna WJ, Gulker H, Krikler DM (1983) The comparative effects of diltiazem and verapamil on atrioventricular conduction and atrioventricular reentry tachycardia. Circ Res 52, 163-168.
- Rudloff E, Raffe MR (2012) Arrhythmias in critical care. In: Proceeding of the Congreso Latinoamericano de Emergencia y Cuidados Intensivos. Mexico.
- Schober KE (2010) Medical Treatment of Tachyarrhythmias. In: Proceedings of the 35th World Small Animal Veterinary Congress. Geneva, Switzerland.
- Schwartz DS (2009) This dog has ventricular arrhythmias-what do I do? In: Proceedings of the 34th World Small Animal Veterinary Congress. Sao Paulo, Brazil.
- Silverstein D (2006) SIRS, MODS, and sepsis in small animals. In: International Congress of the Italian Association of Companion Animal Veterinarians. Rimini, Italy, pp. 107-108.
- Smith LJ (2002) Hypotension. In: S. A. Greene,Veterinary Anesthesia and Pain Management Secrets. Hanley & Belfus Inc, Philadelphia, pp. 135-140.
- Spyer KM (1994) Central nervous mechanisms contributing to cardiovasular control. J Physiol 474, 1-19.
- Stepien RL (2005) Cardiac arrhythmias: What to treat, when and how. In: Proceeding of the North American Veterinary Conference, Orlando, Florida.
- Tag TL, Day TK (2008) Electrocardiographic assessment of hyperkalemia in dogs and cats. J Vet Emerg Crit Care 18, 61-67.
- Tilley LP, Smith WFK (2016) Electrocardiography. In: L. P. Tilley,W. F. K. Smith, J. R. Smith, M. Oyama, M. Sleeper. Manual of Canine and Feline Cardiology. 5th edn. Elsevier, Toronto, pp. 49-77.
- Wendelburg KM, O’Toole TE, McCobb E, Price LL, Lyons JA, Berg J (2014) Risk factors for perioperative death in dogs undergoing splenectomy for splenic masses: 539 cases (2001-2012). J Am Anim Med Assoc 245, 1382-1390.
- William WM (2007) Cardiovascular system. In: V. W. Lumb, E. W. Jones. Veterinary anesthesia and analgesia. 4th edn. Blackwell Publishing, Oxford, pp. 61-116.
Corresponding author:
Zoe Tzenetidou
e-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.



