I.K. Liapis1, Ι. Panopoulos2, D. Psalla3
1DVM - Cert.Ophthalmology
2DVM, PhD, Resident of the European College of Veterinary Diagnostic Imaging (ECVDI)
3DVM, PhD, Laboratory of Pathology, School of Veterinary Medicine, Faculty of Health Sciences, A.U.Th.
Keywords:
lacrimal gland, tumour, orbit, dog
Αbstract
The aim of the present report is to describe the clinical signs, diagnostic evaluation, surgical management and follow-up of a tumour of lacrimal gland origin in a dog. The dog was admitted due to mild exophthalmos and upper eyelid oedema. The physical and ultrasonographic examination of the eye, as well as the cytological examination findings of aspirate obtained with ultrasonographic guidance, led to the diagnosis of orbital neoplasia. During computed tomography of the orbit, it was revealed that the mass was located in the superorostral aspect of the orbit, in the anatomical location of the lacrimal gland. The mass was surgically excised via extensive transfrontal orbitotomy, as described by Hakansson. Histopathology of the mass revealed lacrimal gland adenoma. The dog remains asymptomatic 3 years after surgery. The primary tumours of the orbit are uncommon in dogs and lacrimal gland tumours are rare. In cases of timely diagnosis, surgical excision is the treatment of choice. The surgical techniques that provide extensive exposure of the orbit offer the possibility of excising these tumours in such a way as to preserve the integrity of the eye and optical nerve, thus ensuring that vision is maintained.
Introduction
Τumours are the most common disorder of the orbit (OR) in dogs.1,2 The most common tumours of the orbit include adenocarcinoma, sarcoma, meningioma and mast cell tumor.1-6 Primary tumours originate from any structure of the OR whereas secondary tumours can occur from metastasis or, more often, from tumour infiltration into adjacent tissues.1 Lacrimal gland tumours are rare in dogs.7-10 The clinical signs that manifest with orbital tumours are exophthalmos, conjunctival hyperaemia and congestion, strabismus and third eyelid protrusion whereas digital retropulsion of the globe is limited or impossible.11 Depending on tumour localisation and pressure exerted on the optic nerve, visual loss may be present. 12 Lesions are usually painless. The differential diagnosis of orbital tumours in dogs includes other space-occupying and inflammatory conditions of the OR, such as inflammation and retrobulbar abscess, orbital cellulitis, extraocular myositis, orbital cysts and vascular anomalies.1,11 The diagnosis is based on the history, clinical signs and ultrasonographic findings, as well as computed tomography and magnetic resonance imaging of the OR.1 Current techniques of three-dimensional digital imaging and three-dimensional printing may also contribute to OR imaging and preoperative planning.13 Depending on the results of diagnostic imaging, in order to reach an accurate tumour diagnosis, fine needle aspirate cytological examination, or tru-cut biopsy samples for histopathologic examination is recommended. Ultrasonography and computed tomography offer an opportunity for targeted and safe sampling.1,14,15
Isolated orbital tumours not metastasised or infiltrated can be removed surgically, preserving the integrity of the eye and ensuring vision.1,16-19 Several surgical approaches to the OR and retrobulbar space have been described and applied. Among others, the lateral orbitotomy techniques offer excellent access to the OR.20,21 Recently, the three-step Hakansson technique has been recommended and applied, because, as it combines lateral with transfrontal orbitotomy, it provides extensive orbital and retrobulbar exposure.22
Case Report
A 9-year-old, male crossbreed Poodle was admitted with mild enlargement of the left eye (OS). The dog was in good physical condition, and during physical examination nothing abnormal was found, except mild enlargement of the submandibular and retropharyngeal lymph nodes. During ophthalmologic examination, mild exophthalmos OS, oedema and upper eyelid skin erythema were noted (Figures 1, 2). Strabismus and third eyelid (TE) protrusion were not found, and mouth opening was not painful. Digital retropulsion of the globe OS was not possible. Upper eyelid lifting of OS, revealed a dark, soft tissue mass under the orbital conjunctiva, between the globe and upper orbital rim (Figure 3). The eye was functional OS. Menace response and cotton ball test were positive OS. Pupillary light reflex, both direct and indirect, as well as chromatic pupillary light reflex were normal. Slit-lamp examination revealed no abnormal findings. Schirmer tear test values were 18 mm/min OD (right eye) and 20 mm/min OS. Intraocular pressure was 18 mmHg OD and 19 mmHg OS. Indirect ophthalmoscopy revealed no significant abnormalities. The opposite eye was completely normal.
 |
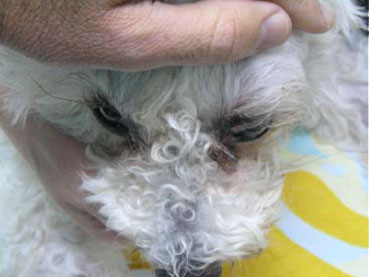 |
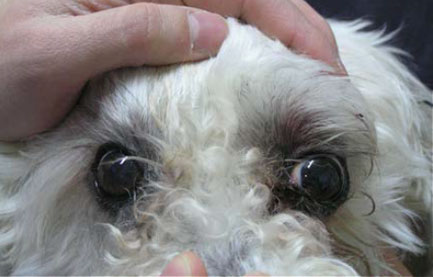
| Figure 1. Ophthalmologic examination at the time of admission. Mild exophthalmos can be perceived only by the larger segment of the sclera of the left eye, which has become visible compared to the right eye. Note the upper eyelid oedema of the left eye, the skin erythema, and the absence of strabismus and TE protrusion. | Figure 2. Ophthalmologic examination at the time of admission. Mild exophthalmos can be perceived if the head is observed from above. Observation from this point of view also differentiates exophthalmos from buphthalmos due to glaucoma, in which case the globe does not protrude visibly. |
In ophthalmic ultrasonography (Esaote PA023 microonvex 10 MHz transducer), a mass of mixed echogenicity was revealed. The mass which was mostly echogenic, large, compact and with indistinct multilobulated periphery, occupied most of the retrobulbar space (Figure 4).
 |
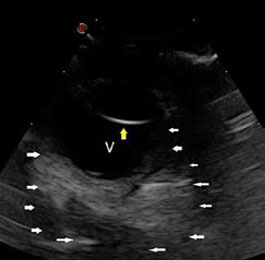 |
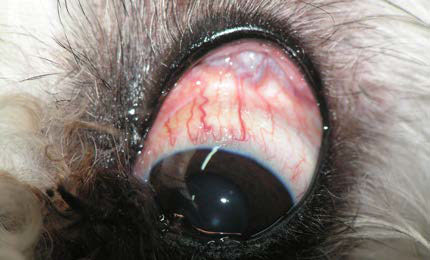
| Figure 3. Left eye examination at the time of admission. A dark soft tissue mass is observed under the orbital conjunctiva between the globe and the upper orbital rim. | Figure 4. Ophthalmic ultrasonography. A mass of mixed echogenicity, mostly echogenic, large, compact and with indistinct multilobulated periphery, is visualised in the retrobulbar space (white arrows). The yellow arrow indicates the posterior lens capsule and V indicates the vitreous humour. |
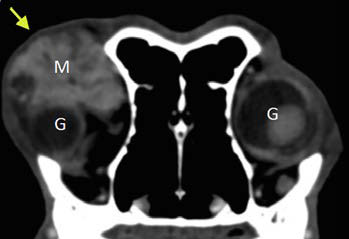 |
| Figure 5. Computed tomography of the OR after intravenous infusion of contrast agent. A space-occupying, well-defined mass with increased signal density (Μ) is revealed at the superorostral aspect of the OR, in the anatomical location of the left lacrimal gland (yellow arrow). Note the displacement of the globe OS into the OR compared to the contralateral healthy eye due to pressure exerted by the mass. |
Ultrasound-guided fine needle aspiration (FNA), revealed atypical polygonal cells with eccentric nuclei and foamy cytoplasm. Retrobulbar neoplasia was diagnosed. For accurate lesion localisation and visualisation of tumour margins, multi-slice helical computed tomography (CΤ) was performed, with 1.25 mm slices, prior to and post intravenous infusion of non-ionic iodinated contrast agent, via an automated infusion pump, at an infusion rate of 3 ml kg-1 and contrast agent concentration of 600 mgI kg-1. A large (38 Χ 28 Χ 16 mm) mass with soft-tissue attenuation (57 HU) was revealed, with marked heterogeneous signal enhancement pattern (160 HU) after contrast agent infusion. The mass was located in the superorostral space of the orbit, in the anatomical location of the lacrimal gland, which could not be visualised (Figure 5), and it expanded in the retrobulbar space, exerting pressure on adjacent tissues and displacing the eyeball anterio-ventrally. (Figures 6A and 6B). The regional bone structures, the unilateral zygomatic salivary gland and the rest of the anatomical structures of the OR were normal (Figure 7). The regional lymph nodes (submandibular and retropharyngeal) were bilaterally normal, slightly enlarged and with homogeneous mixed contrast enhancement patterns on CT after intravenous infusion of contrast agent. FNA examination of regional enlarged lymph nodes on the side of the affected eye did not reveal any atypical cells. Routine haematology and biochemistry evaluation, as well as thoracic radiographs and abdominal ultrasonography were normal.
Surgical excision of the mass was undertaken. Pre-medication was performed with midazolam (Dormicum, Roche Pharma, Germany) [0.2 mg kg-1 intravenously, (IV)], fentanyl (Fentanyl, Janssen, FAMAR, Hellas) (starting dose 2 μg kg-1 IV and maintenance dose 2 μg kg-1 hr-1 in constant rate infusion) and meloxicam (Metacam, Boehringer Ingelheim, Spain) [0.1 mg kg-1 subcutaneous (SC), once a day, (SID)]. Amoxicillin/clavulanic acid was administered (Synulox RTU, Zoetis, Hellas) (20 mg kg-1 SC, SID) for antibiotic therapy. Induction of anaesthesia with propofol (Propofol MCT/LCT/Fresenius 1%, Fresenius Kabi, Austria) (4 mg kg-1 IV) was followed by maintenance with inhaled isoflurane (Iso-Vet, Piramal Healthcare, UK) after endotracheal intubation. Due to the location and size of the mass, surgical approach was performed by the three-step transfrontal orbitotomy described by Hakansson. Skin incisions are made bilaterally parallel to that segment of the facial nerve so that it can be preserved intact (Figures 8-12).
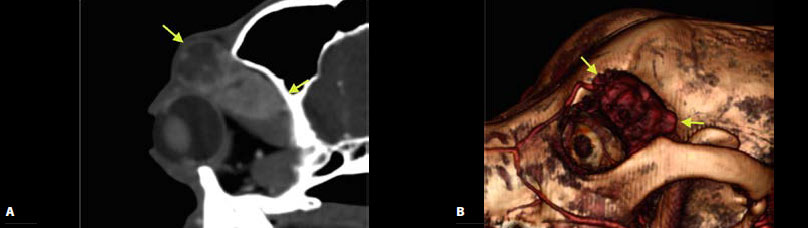 |
| Figure 6. Computed tomography after intravenous infusion of contrast agent in sagittal section of the OR (A) and three-dimensional reconstruction images (B). Presence of a space-occupying mass in the superorosral aspect of the OR, in the anatomical location of the left lacrimal gland (yellow arrows) and expansion of the mass in the retrobulbar space. |
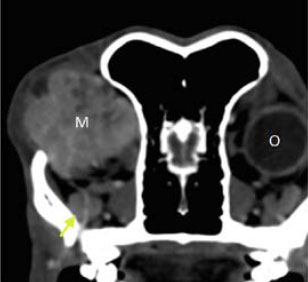 |
Figure 7. Transverse computed tomography image of the retrobulbar space after the intravenous infusion of contrast agent. The presence of a well-defined space-occupying mass with increased density (Μ) that expands into the left retrobulbar space is noted. Compared to the healthy right eye the globe of the left eye (O) cannot be visualised in this scan due to anterior displacement of the left eye by the mass. The regional bone structures were smooth in outline and the zygomatic salivary gland (yellow arrow). |
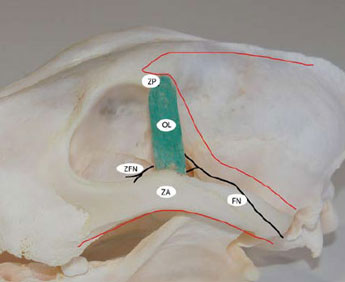 |
Figure 8. Schematic representation of the skin incisions and nerves of the OR area. The red lines mark the skin incisions and the black lines indicate nerves. ΖA: Ζygomatic arch, OL: Orbital ligament, ZP: Zygomatic process of the frontal bone, FN: Facial nerve, ZFN: Ζygomaticofacial nerve. |
First step: Skin incisions and resection of the zygomatic arch (ΖA)
The first incision was made along the ventral aspect of the zygomatic arch (ZA). The second incision was made along the dorsal aspect of the ZA, and was directed cranially and medially, parallel to the orbital rim and extended posteriorly in a curvilinear fashion, parallel to the midline of the skull. Between the two incisions, an intact flap of skin, subcutaneous tissues, and muscle fascia extending from the posterior portion of the ZA to the lateral canthus was preserved. The facial nerve was included within this flap and preserved intact (Figure 8). The skin and subcutaneous tissue of the initial incision were deflected dorsally to reveal the ZA. The aponeurosis of the temporalis muscle was incised along the dorsal edge of the ZA, and along the orbital rim rostral to the orbital ligament (OL). Thereafter, the OL was resected at its point of insertion in the ZA. During resection, the zygomaticofacial nerve was preserved and a small segment of the OL was left on the ZA in order to facilitate suture placement at closure. Prior to ZA osteotomy, two holes for cerclage wires were pre-drilled by orthopaedic bone drill, bilateral to the planned osteotomy sites in preparation for reconstruction of the orbit prior to closure. Osteotomy of the ZA was accomplished using an oscillating bone saw. After osteotomy, the mobilised portion of the ZA with the attached masseter muscle was deflected ventrally (Figure 9).
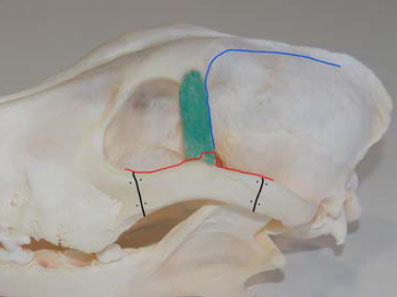 |
Figure 9. Schematic representation of zygomatic process osteotomy and incision of the temporalis muscle aponeurosis. The black lines mark the osteotomy sites and the dots indicate the drill holes for cerclage in the zygomatic arch. The red line indicates the pattern of incision in the aponeurosis of the temporalis muscle along the ZA, the orbital ligament and the ventral aspect of the orbital rim (mentioned from caudal to rostral direction). The dotted blue line indicates the incision into the aponeurosis of the temporalis muscle along the orbital ligament, the zygomatic process of the frontal bone and the external sagittal crest. |
Second step: Elevation of the temporalis muscle
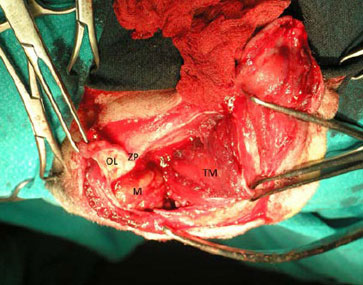
During the second incision, the skin, subcutaneous tissues and the frontalis muscle were incised and caudally deflected, and the aponeurosis of the temporalis muscle was incised along the caudal edge of the OL and then along the temporal line, in a caudal direction along the external sagittal crest (Figure 9). After it was separated from other masticatory muscles, the temporalis muscle was elevated bluntly and retracted caudally. During this step, most of the outer-lateral aspect of the mass was exposed and the third step of the procedure was undertaken for secure excision of the mass under direct visualisation of the cranial and medial aspect of the mass (Figure 10).
 |
 |
| Figure 10. Intraoperative photograph showing surgical access of the OR after the second step was completed and prior to zygomatic process osteotomy. The rostral aspect of the dog’s muzzle is on the left side. OL: Orbital ligament, ZP: Zygomatic process, M: Mass, TΜ: Temporalis muscle. | Figure 11. Schematic representation of zygomatic process (ZP) osteotomy. The black line indicates the osteotomy site and the dots indicate the drill holes for cerclage wires on the ZP. |
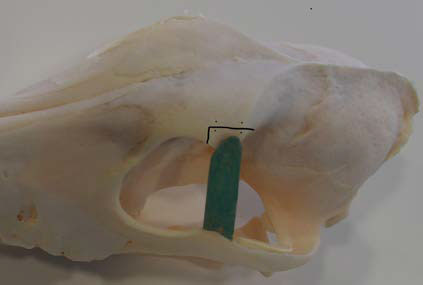
Τhird step: Zygomatic process (ZP) osteotomy
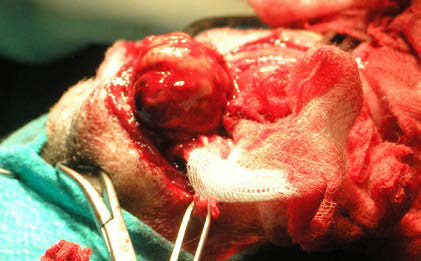
Prior to ZP osteotomy, two drill holes for cerclage wires were made by orthopaedic bone drill bilateral to the osteotomy site. Thereafter, osteotomy was performed in two cuts perpendicular to one another by oscillating bone saw (Figure 11). After osteotomy, the ZP along with the OL, the dorsal orbital septum and the upper eyelid were all deflected rostrally, thus ensuring surgical access to almost the entire orbital cavity and extensive exposure of the mass which was then removed intact after surgical separation from adjacent tissues (Figures 12, 13).
 |
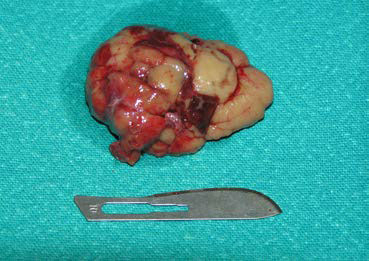 |
| Figure 12. Intraoperative photograph showing mass excision after extensive exposure of the OR and surgical manipulation of most of the orbital tissues. The rostral aspect of the dog’s muzzle is on the left side. | Figure 13. Photograph of the mass taken after removal. |
Surgical reconstruction of the ΟR followed. The ZP and the ΖA were repositioned and reconstructed with cerclage wire 0.8 mm in diameter. The OL and the aponeurosis of the temporalis muscle were sutured with 3/0 absorbable monoclonal sutures. Finally, the frontalis muscle, subcutaneous tissues and skin were sutured in place and closed routinely.
 |
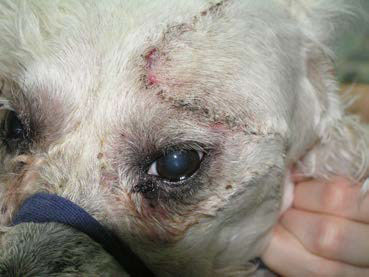 |
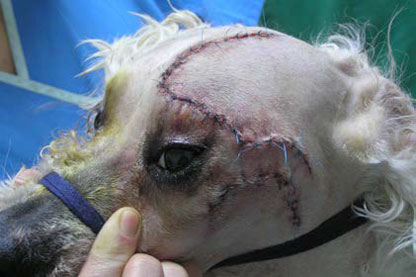
| Figure 14. Photograph of the dog taken after recovery from anaesthesia. | Figure 15. Photograph of the dog taken on the 10th day postoperatively after suture removal. |
Anaesthetic recovery and the immediate post-surgical period were uneventful (Figure 14). Analgesic and antibiotic administration continued throughout the duration of hospitalisation. Two days after the surgery the dog was discharged. Analgesics and antibiotics were continued for five more days. Ten days after the surgery the skin sutures were removed. At that time point, physical examination was normal, exophthalmos was no longer present and vision was normal (Figure 15). Schirmer tear test values were 19 mm min-1 OD (right eye) and 17 mm min-1 OS. Histopathological examination of the mass revealed lacrimal gland adenoma (Figure 16). Three years after surgery the dog remains asymptomatic, vision is normal and tear production in the OS has remained consistent and within normal range.
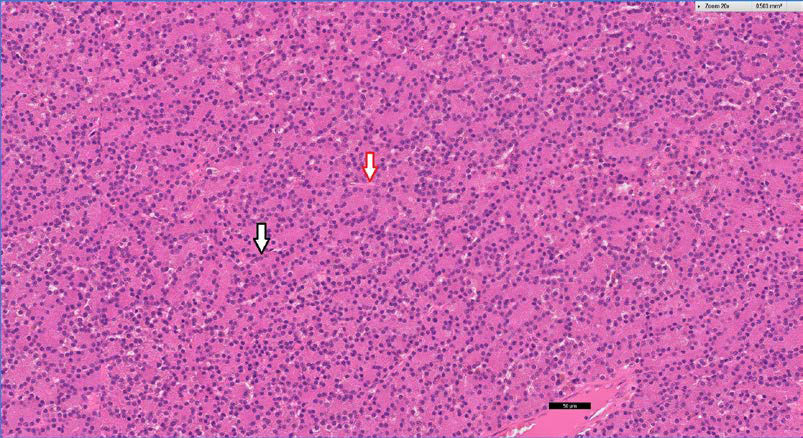 |
| Figure 16. Lacrimal gland adenoma. Neoplastic tissue is composed mainly of cuboidal and polygonal cells with abundant eosinophilic cytoplasm and round nuclei that form tightly fitted tubuloalveolar structures (black arrow). The thin fibrovascular membrane is marked by the red arrow. Stained by haematoxylin and eosin. Microscopic image (Χ20). |
Discussion
Exophthalmos is a characteristic clinical sign of orbital disorders in dogs. It should be differentiated from glaucoma, in which enlargement of the globe can be perceived as exophthalmos. When in doubt, observation of the head from above (Figure 2) and, especially, measurement of intraocular pressure (IP) can differentiate the two disorders.11 In exophthalmos, IP is normal or marginally increased compared to the contralateral healthy eye. In this particular case, IP of the left eye was within normal range and similar to that of the healthy right eye. Other clinical signs of orbital disorders include oedema and conjunctival hyperaemia, strabismus, TE protrusion, decreased retropulsion of the globe, and pain that is elicited during palpation of periocular tissues, as well as during opening of the mouth.11 Pain is a feature of acute and inflammatory disorders of the OR, whereas it is usually less severe or absent in chronic and neoplastic disorders.1 From the above clinical signs, strabismus and TE protrusion, which are typical clinical signs of orbital tumours were absent in this case, despite the large size of the mass. The absence of strabismus can be attributed to the distribution of the mass in the OR in such a way that pressure was exerted in a uniform manner on the globe and the absence of TE protrusion could be attributed to the expansion of a segment of the mass, mostly dorsal to the globe, between the periorbital fascia and the dorsal rim of the OR, in such a way that the eye was displaced in a downward direction, preventing the protrusion of the TE.
Diagnosis of orbital neoplasia should not be based only on the findings of physical examination. In a retrospective study of 44 cases with orbital neoplasia, 1/3 of cases had an acute manifestation with pain and purulent discharge, clinical signs consistent with inflammatory disorders.4 For this reason, diagnostic imaging is necessary for further investigation of orbital disorders. Among others, ocular ultrasonography is the gold standard because it is an easy, safe, and quick examination which does not require general anaesthesia. However, the images obtained are two-dimensional and ultrasonography findings are non-specific in most cases.1 Cytological examination and/or histopathology are necessary for differentiating between neoplasia and inflammation, whereas cases have been reported, in which immunohistochemistry analysis of the mass is necessary for the definitive diagnosis.23 In the case described in the present report, ocular ultrasonography revealed the presence of a retrobulbar space-occupying lesion whereas cytological examination of samples obtained from the lesion site showed atypical neoplastic cells that confirmed the diagnosis of an orbital tumour.
Nowadays, CT is the imaging modality of choice for the detailed localisation of OR lesions and evaluation of local anatomical structures.1 CT can also be useful for guided biopsy sample from the mass.15 Finally, with three-dimensional reconstruction and most recently with three-dimensional printing, CT can significantly contribute to the selection of surgical technique for orbital tumour management.13 In our case, computed tomography and three-dimensional digital reconstruction of the OR defined the exact dimensions of the mass and the precise position within the OR, explaining as previously mentioned the absence of strabismus and TE protrusion. Furthermore, CT excluded the expansion of the mass to the bones of the OR, the globe, the optical nerve and the extraocular muscles. Magnetic resonance imaging is preferable in the cases that detailed imaging of soft tissues of the OR and mainly of the optic nerve, are necessary.1,4
Orbital tumours can be surgically excised in the case of early diagnosis, as long as they have not yet metastasised or infiltrated adjacent anatomical structures of the OR. Surgical excision can be accomplished via anterior approach and exenteration, in cases when vision is no longer present or via orbitotomy when the eye is still functional.1,16-19 In the case of the present report, the investigation for metastases in the thoracic and/or abdominal cavity was negative. Cytological examination of regional lymph nodes was also negative for malignancy. Mild enlargement of these lymph nodes, which was bilateral, was attributed to reactive regional lymphadenopathy due to mild chronic gingivitis. Mass removal after orbitotomy was undertaken so that vision could be preserved.
According to CT findings, the mass was located in the superorostral space of the OR, where the lacrimal gland is located, expanding into the retrobulbar space and exerting pressure on adjacent tissues. For that reason, transfrontal orbitotomy was applied as it was described by Hakansson. The lateral and modified lateral orbitotomy techniques have been also described.20,21 Compared to the latter, the transfrontal orbitotomy that was selected in this case, ensured the visual evaluation of all the aspects of the mass, because it provided more extensive access to the anterior dorsal and medial aspect of the OR.22 By the time the 2nd step of the procedure was completed, the retrobulbar space and most of the mass became accessible; however, the completion of the technique was undertaken with osteotomy and cranial traction of the ZP, so as to provide extensive access to the lacrimal gland which, according to the computed tomography findings, had an anatomical connection to the mass. In this manner, the cranial and medial aspect of the mass was surgically separated from the surrounding tissues under direct visualisation and the mass was excised along with the lacrimal gland.
Malignant tumours are the most common disorder of the OR in dogs. Tumours of mesothelial origin (osteosarcoma, fibrosarcoma, chondrosarcoma, rhabdomyosarcoma) and of epithelial origin (adenocarcinoma, squamous cell carcinoma) are most commonly reported.1-5 They usually originate from adjacent neoplastic tissues, usually from the nasal cavity, the frontal and paranasal sinuses and muscles. From the rest of the tumour types, meningioma, mast cell tumour and lymphoma have been reported.1 Among the benign tumours canine lobular orbital adenoma has been reported.24 Tumours of lacrimal gland origin are rare in dogs.25 Few cases of lacrimal gland adenoma and adenocarcinoma have been reported originated both from the main lacrimal gland8-10 as well as the accessory lacrimal gland of the third eyelid.7,18,19 In this particular case, clinical signs, diagnostic imaging characteristics and cytological examination were consistent with a lacrimal gland tumour. Intraoperative differentiation from lobular orbital adenoma was based on the macroscopic appearance of the mass which was well-defined (Figure 13). Lobular orbital adenoma is multilobulated and expands between anatomical structures within the OR, thus preventing its full removal, leading to frequent relapse.24 Ηistopathological examination of the mass confirmed the diagnosis of adenoma. Frequently, the differentiation between adenoma of the lacrimal and zygomatic salivary gland, is not possible with standard histopathology only.7 In such cases, the definitive diagnosis is accomplished with histochemistry and immunohistochemistry analyses.26 In the present case the application of such modalities was not considered necessary because, according to computed tomography, the zygomatic salivary gland was normal, the mass had an anatomical connection to the lacrimal gland and histopathological examination revealed that the mass was composed by serous neoplastic cells of adequate differentiation, which permitted the identification of the tumour origin.
Despite removal of the entire lacrimal gland, there was no reduction in tear production immediately after the surgery, as well as in the three years that followed, during which the dog was monitored. This can be attributed to the fact that, in many animals, most of the aqueous layer of tears is produced by the accessory lacrimal gland of the third eyelid and not from the main gland. Also, it has been proven that, in cases where one of the two lacrimal glands has been removed, the remaining gland develops a reactive increase in tear production.25 Successful tumour excision, preservation of vision and longterm survival of the dog can mostly be attributed to the benign nature of the tumour, as well as early diagnosis and timely surgical management, a fact that is in agreement with the data of the literature, where it is reported that timely excision of orbital tumours in dogs, while vision is still present and prior to infiltration of adjacent tissues or manifestation of metastases, may preserve vision and increase the survival rate.
References
1. Spiess BM, Pot SA. Diseases and surgery of the canine orbit. In: Veterinary ophthalmology. Gelatt KN, Gilger BC, Kern TJ (ed). 5th edn. John Wiley & Sons Inc: Iowa, 2013, pp.793-831.
2. Armour MD, Broome M, Giuseppe DA, Blades NJ, Esson DW. A review of orbital and intracranial magnetic resonance imaging in 79 canine and 13 feline patients (2004-2010). Vet Ophthalmol 2011, 14:215-226.
3. Attali-Soussay K, Jegou JP, Clerc B. Retrobulbar tumors in dogs and cats: 25 cases. Vet Ophthalmol 2001, 4:19-27.
4. Hendrix DV, Gelatt KN. Diagnosis, treatment and outcome of orbital neoplasia in dogs: a retrospective study of 44 cases. J Small Anim Pract 2000, 41:105-108.
5. Labelle AL, Labelle P. Canine ocular neoplasia: a review. Vet Ophthalmol 2013, 16, Supplement 1:3-14.
6. Scott EM, Teixeira LBC, Flanders DJ, Dubielzic RR, McLellan GJ. Canine orbital rhabdomyosarcoma: a report of 18 cases. Vet Ophthalmol 2016, 19:130-137.
7. Kern TJ. Orbital neoplasia in 23 dogs. J Am Vet Med Assoc 1985, 186:489-491.
8. Fun IW, Chao TT, Yi SL. Orbital adenocarcinoma of lacrimal gland origin in a dog. J Vet Diagn Invest 2001, 13:159-161.
9. Hirayama K, Kagawa Y, Tzuzuki K, Azuma Y, Yoshino T, Taniyama H. A pleomorphic adenoma of the lacrimal gland in a dog. Vet Pathol 2000, 37:353-356.
10. Schappa JT, Peterson A, Dubielzig RR, Lim CC, Overmann JA. What is your diagnosis? Upper eyelid swelling in a dog. Vet Clin Pathol 2014, 43:111-112.
11. Mound JRB. Conditions of the orbit and globe. In: Manual of small animal ophthalmology. Simon M. Petersen J, Crispin SM (ed). 1st edn. BSAVA: West Sussex, 1993, pp.45-64.
12. Mauldin EA, Deehr AJ, Hertzke D, Dubieltzic RR. Canine orbital meningiomas: a review of 22 cases. Vet Ophthalmol 2000, 3:11-16.
13. Three-dimentional printing of orbital and periorbital masses in three dogs and its potential applications in veterinary ophthalmology. Vet Ophthalmol 2017, 20:58-64.
14. Boydell P. Fine needle aspiration biopsy in the diagnosis, of exophthalmos. J Small Anim Pract 1991, 32:542-546.
15. Cirla A, Rondena M, Bertolini G. Automated tru-cut imaging-guided core needle biopsy of canine orbital neoplasia. A prospective feasibility study. Open Vet J 2016, 6:114-120.
16. Bartoe JT, Brightman AH, Davidson HJ. Modified lateral orbitotomy for vision-sparing excision of a zygomatic mucocele in a dog. Vet Ophthalmol 2007, 10:127-131.
17. Lassaline ME, Gelatt KN, Brooks DE, Ellison GW. Orbitotomy for retrobulbar malignant fibrous histiocytoma in a dog. Vet Ophthalmol 2005, 8:1-6.
18. Michaud B, Manville S. Extraction d’une tumeur retro-orbitaire par orbitotomie transfrontale. Le Point Veterinaire 2016, 370:54-61.
19. Gilger BC, Whitley RD, McLaughlin SA. Modified lateral orbitotomy for removal of orbital neoplasms in two dogs. Vet Surg 1994, 23:53-58.
20. Slatter DH, Abdelbaki Y. Lateral orbitotomy by zygomatic arch resection in the dog. J Am Vet Med Assoc 1979, 175:1179-1182.
21. Gilger BC, Whitley RD, Mclaughlin SA. Modified lateral orbitotomy for removal of orbital neoplasms in two dogs. Vet Surg 1994, 23:53-58.
22. Hakansson NW, Hakansson BW. Transfrontal orbitotomy in the dog: an adaptable three-step approach to the orbit. Vet Ophthalmol 2010, 13:377-383.
23. Rzechorzek MN, Smith C, Schwarz T, Liuti T, Elders R, Woodw S, Lawrence J, Marioni-Henry K. Idiopathic sclerosing orbital inflammation mimicking a malignant spindle cell tumor in a dog. Clin Case Rep 2016, 4:935-943.
24. Headrick JF, Ellison B, Dubielzig RR.Canine lobular orbital adenoma: a report of 15 cases with distinctive features Vet Ophthalmol 2004, 7:47-51.
25. Giuliano EA. Diseases and surgery of the canine lacrimal secretory system. In: Veterinary ophthalmology. Gelatt KN, Gilger BC, Kern TJ (ed). 5th edn. John Wiley & Sons Inc: Iowa, 2013, pp.912-944.
26. Giudice C, Marco R, Mirco R, Luca M, Giorgio C. Zygomatic glandadenoma in a dog:histochemical and immunohistochemical evaluation. Vet Ophthalmol 2005, 8:13-16.
Corresponding author:
Ιgnatios Liapis
31 Al. Panagouli Str.
153 43 Ag.Paraskevi, Attica
Tel: +30 210 6082308-9
e-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.



