N.D. Tsimitris1, M.Ι. Kouki2, G.D. Brellou3, L.G. Papazoglou4, T. Slini5, P. Papadopoulou6, S.A. Papadimitriou7
1DVM, MSc
2DVM, PhD
3DVM, PhD, Laboratory of Pathology, School of Veterinary Medicine, Faculty of Health Sciences, A.U.Th.
4DVM, PhD, MRCVS, Companion Animal Clinic, School of Veterinary Medicine, Faculty of Health Sciences, A.U.Th.
5Mathematician, PhD, Department of Mechanical Engineering, A.U.Th.
6DVM, PhD, Companion Animal Clinic, School of Veterinary Medicine, Faculty of Health Sciences, A.U.Th.
7DVM, DDS, PhD, Companion Animal Clinic, School of Veterinary Medicine, Faculty of Health Sciences, A.U.Th.
Keywords:
neoplasm, dog, oral cavity
Abstract
Medical records of 63 dogs admitted with oral neoplasms at the Companion Animal Clinic, School of Veterinary Medicine of the Aristotle University of Thessaloniki over a 10-year-period (2005-2014) were reviewed. The objective of this retrospective study was to describe the long-term outcome associated with curative-intent surgery, and the prevalence of the most common oral neoplasms in relation to the population characteristics of dogs presented at the clinic. Fifteen different types of neoplasms were recognised. The majority of the population was male adult, purebred dogs. Male predilection was found for fibrosarcomas, peripheral odontogenic fibromas, acanthomatous ameloblastomas, and oral papillomas.The most commonly occurring malignant neoplasms were malignant melanomas, fibrosarcomas, squamous cell carcinomas and undifferentiated pleomorphic sarcomas. Τhe most frequently encountered benign neoplasms were canine acanthomatous ameloblastomas, peripheral odontogenic fibromas and viral papillomatosis. The majority of tumors occurred on the mandibular and maxillary gingiva. Bone invasion was radiographically observed in 65.71% of the cases. Regional lymph node metastasis was very common among malignant melanomas, but for the rest type of the tumours data is not sufficient. Mandibulectomy or maxillectomy were the treatment of choice for the malignant infiltrating types and for acanthomatous ameloblastomas. Complications were recorded in 34.9% of all cases and were classified either as major or minor. Local recurrence was mostly observed in fibrosarcomas. Curative-intent surgery was able to provide a sufficient cause specific survival, even though a large number of animals were initially presented with masses of substantial size, according to WHO staging.
Introduction
Oral neoplasms account for 6-7% of canine cancer cases and is the most common neoplasm overall, after the skin, the mammary, the rest of the digestive and the haemolymphatic systems.1,2 Oropharyngeal neoplasms are 2.6 times more common in dogs than in cats, and male dogs have a 2.4 times greater risk for developing oropharyngeal malignancy compared to females.3,4 The incidence of the neoplasms increases after the age of 8, with a mean of 9.8 years.4,5
Oral neoplasms may present, not only as a typical mass, but also as a non-healing, ulcerated lesion. They can originate from the gingiva, dental structures, tonsils, buccal or labial mucosa, hard or soft palate and tongue, spreading through direct expansion or invasion of the adjacent bone and cartilaginous tissue.6,7 The most common site of oropharyngeal neoplasia is the gingival mucosa followed by the tonsils and the labial/buccal mucosa.6
The most common oral neoplasms in dogs include malignant melanoma (MM) (31-42%), squamous cell carcinoma (SCC) (17-25%), fibrosarcoma (7.5- 25%) and osteosarcoma (6-18%). Considering the benign types, acanthomatous ameloblastoma (AA) and peripheral odontogenic fibroma (POF) are the most common.8,9 The actual incidence among the benign neoplasms is unknown due to confusion over nomenclature and the fact that many clinicians do not routinely submit specimens for histopathological identification.9,10
During the past years, novel treatments have been applied as adjuvant therapies for cancer in companion animals. Nevertheless, surgery remains the mainstay in the treatment of most oral neoplasms.11,12 The primary goal of surgery is to achieve clean surgical margins, and to maintain normal function and cosmesis.13
The objective of the present study was to describe retrospectively the prevalence of oral neoplasms and the long-term follow-up after surgical excision, in dogs presented to a university hospital.
Materials and methods
Medical records of 63 dogs with oral neoplasms that were surgically excised were retrieved from the archives of the Companion Animal Clinic, Aristotle University of Thessaloniki, from January 2005 through December 2014. Only neoplasms that had a histological diagnosis were included. Benign growths such as gingival hyperplasia and infectious conditions, cases not amenable to surgery due to the size of the tumour, animals that had received neo-adjuvant treatment prior to presentation or when the owner declined surgery (financial restraints, reluctance to proceed with salvage techniques) were excluded from this study. Metastatic neoplasms of the tonsils were not categorised as a separate clinical entity. Records were reviewed to obtain signalment, body weight, tumour size, modality of treatment, lymph node (LN) metastasis, radiographic examination, histopathology results, complications and follow-up/outcome. Thoracic radiographs were evaluated for signs of metastasis and skull radiographs for bone invasion. Surgical margins were not considered because they were not available in a sufficient number of cases.
Disease-free interval (DFI) was calculated from the day of surgery to the first day of detected local macroscopic recurrence. Follow-up information was obtained through medical records or phone contact with the owner. Animals that outlived 365 days after curative-intent surgery were considered to have a good outcome.
Median age was calculated, and gender and breed distribution were summarised by frequencies and percentages. A Spearman correlation analysis was performed to assess any relation between tumour types, incidence and site of appearance. Statistical analysis was performed with the SPSS software pack age. Significant correlations were flagged at the 0.01 (2-tailed) and 0.05 (2-tailed) levels respectively.
All samples were fixed in 10% buffered formalin for 2 days and routinely processed for histopathological diagnosis. Paraffin sections of 3 mm were stained with hematoxylin and eosin. Immunohistochemistry was not performed in any case.
*Acanthomatous ameloblastoma,
†Peripheral odontogenic fibroma, ‡Malignant melanoma,
§Squamous cell carcinoma, #Undiferentiated pleomorphic sarcoma
£Segmental or subtotal or hemi-mandibulectomy /partial or hemimaxilectomy
Results
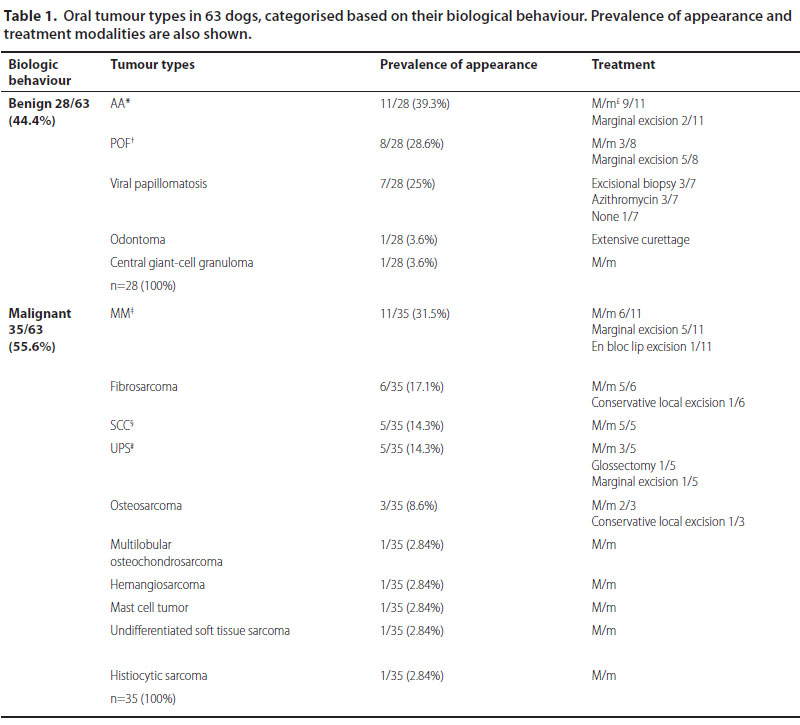
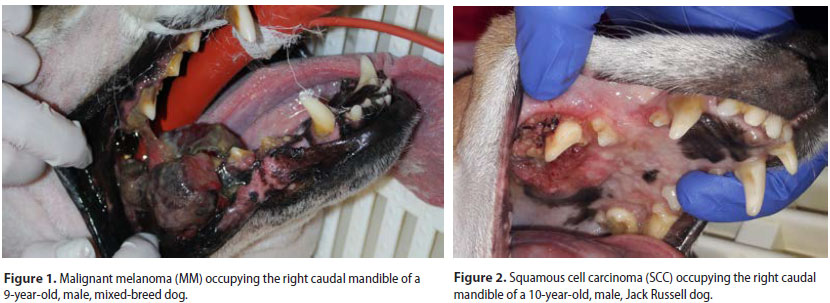
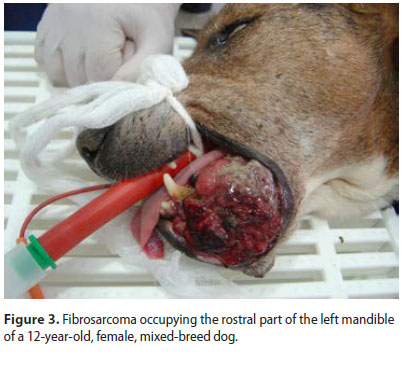
Sixty-three dogs of which 36 males and 27 females were enrolled. Median age at the time of diagnosis was 9.2 years (range: 5 months-17 years). Median age at the time of diagnosis was 8.5 years for the malignant and 9.9 years for the benign neoplasms, respectively. Thirty-nine dogs were purebreds, 6 were crossbreeds and 18 were of mixed-breed. Ten malignant and 5 benign types were recognised (Table 1) (Figures 1-3). Breed predilection could not be assessed due to the relatively small sample size and disparity between tumour types. Sex predilection was noticed for the benign neoplasms (male:female, 2.5:1) but not for malignancies (0.85:1, respectively) except for fibrosarcomas. More specifically, male predilection was found for fibrosarcomas, POFs, AAs, and oral papillomas. The tumour location, and the WHO clinical staging are presented in Table 2. The most common tumour location was the caudal mandible (rs=0.400, p=0.003). In addition, the size of the tumour was significantly associated with the outcome (rs=0.481, p=0.000), but not with the mortality (rs=0.1670, p=0.196).
Regional LNs were evaluated, for regional metastasis by means of fine needle aspiration or excisional biopsy (23/63). Radiographs were taken to assess local bone involvement in 35 dogs and thoracic metastasis in 40 dogs. Data concerning LNs and radiographic evaluation were not available for all cases due to the retrospective nature of the study. Lymph node metastasis was detected in 77.7% (7/9) of the MM and 50% (1/2) of the fibrosarcomas for which data were available. Infiltration was also significantly associated with the outcome (rs=0.301, p=0.016). The incidence of LN infiltration, bone invasion and thoracic metastasis are shown in Table 2.
*Acanthomatous ameloblastoma, †Peripheral odontogenic fibroma, ‡Malignant melanoma, §Squamous cell carcinoma, #Undiferentiated pleomorphic sarcoma
N/A: not applicable
T1: <2 cm maximum diameter, T2: 2-4 cm maximum diameter, T3: >4 cm maximum diameter
L/N: Lymph node
 Mandibulectomy or maxillectomy was performed in all cases of malignancy and in most cases of AA. The majority of animals were presented with ≥ 2cm diameter neoplasms (T2-T3 according to WHO staging). In order to achieve clear surgical margins, resection was planned based on the presence of osteolysis, size of the neoplasm and location. Excision included 1-2 cm gross margins based on i) radiographic findings, ii) gross size of the lesion, iii) size of the jaws. Palliative surgery was performed in 2 cases with thoracic metastasis, but relatively small tumour size, as requested by the owner. Concerning the benign but locally invasive types, 2 cases of AA without evidence of bone involvement were treated with marginal excision due to the owners’ reluctance to proceed in salvage techniques. The majority of the remaining cases were treated mainly with marginal (en block) resection, or mandibulectomy/maxillectomy, as needed. Oral papillomatosis was treated by means of excisional biopsy and/or administration of azithromycin. Regarding the soft tissues, partial glossectomy was performed for lingual neoplasms and wedge en bloc resection followed by reconstruction was applied in a dog with labial MM. Treatment offered is presented in Table 1. Tonsils were affected in 2 cases of MM and 1 undifferentiated pleomorphic sarcoma (UPS). Tonsillectomy was concurrently performed in the 3 cases of tonsillar enlargement.
Mandibulectomy or maxillectomy was performed in all cases of malignancy and in most cases of AA. The majority of animals were presented with ≥ 2cm diameter neoplasms (T2-T3 according to WHO staging). In order to achieve clear surgical margins, resection was planned based on the presence of osteolysis, size of the neoplasm and location. Excision included 1-2 cm gross margins based on i) radiographic findings, ii) gross size of the lesion, iii) size of the jaws. Palliative surgery was performed in 2 cases with thoracic metastasis, but relatively small tumour size, as requested by the owner. Concerning the benign but locally invasive types, 2 cases of AA without evidence of bone involvement were treated with marginal excision due to the owners’ reluctance to proceed in salvage techniques. The majority of the remaining cases were treated mainly with marginal (en block) resection, or mandibulectomy/maxillectomy, as needed. Oral papillomatosis was treated by means of excisional biopsy and/or administration of azithromycin. Regarding the soft tissues, partial glossectomy was performed for lingual neoplasms and wedge en bloc resection followed by reconstruction was applied in a dog with labial MM. Treatment offered is presented in Table 1. Tonsils were affected in 2 cases of MM and 1 undifferentiated pleomorphic sarcoma (UPS). Tonsillectomy was concurrently performed in the 3 cases of tonsillar enlargement.
Overall, post-operative complications were recorded in 34.9% of the cases (22/63) and were classified either as major or minor. Major complications accounted for 13.63% (3/22), including oronasal fistula, extensive wound dehiscence and massive haemorrhage. Minor complications (86.36%, 19/22) involved drooling-associated perioral dermatitis, ranula or seroma formation, difficulty in food prehension and mild drift of the mandible. Information on owners’ satisfaction was available for 41/63 (65%) of the patients, describing the outcome and cosmesis as good to excellent.
 |
|
AA: Acanthomatous ameloblastoma,
POF: Peripheral odontogenic fibroma, MM: Malignant melanoma, SCC: Squamous cell carcinoma UPS: Undifferentiated pleomorphic sarcoma N/A: not applicable |
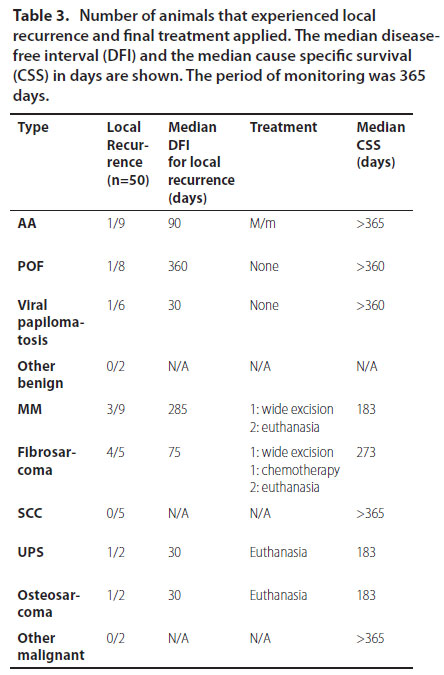
Local recurrence was assessed in 50/63 dogs. Overall, recurrence was noticed in 22% (11/50) of infiltrating types and 1 case of extended papillomatosis, which was initially treated with azithromycin. No relapses were noticed for SCC. Only 3 dogs underwent revision surgery. The rest were either euthanised or no therapy was undertaken, according to the owners’ decision (Table 3).
Discussion
Oral neoplasms accounts for 46% of the canine head and neck cancer. Dog is the most frequently affected species among companion animals.6
In the present study, the gingival mucosa was the most commonly affected site (82.5%). The finding is in accordance with other studies.6 The rest of the oral soft tissues were uncommonly affected, as previously reported.6 Concerning the malignant neoplasms, MM, SCC, fibrosarcoma and osteosarcoma were the most prevalent malignancies, as previously reported.14 Despite the relatively small number of animals, UPS (previously called malignant fibrous histiocytoma)15 and SCC were equally encountered in our study. The latter observation may be biased owing to the strict inclusion criteria. Moreover, immunohistochemistry, which is one of the most important ancillary techniques for the charactersation of neoplastic diseases,16 had not been used in order to accurately differentiate the encountered malignant types.
Acanthomatous ameloblastoma was the most commonly diagnosed benign type in our study, followed by POF and viral papillomatosis with almost equal incidence. Epidemiological data reported previously regarding the abovementioned tumours is inconsistent, so a direct comparison of the results is not possible.10 However, AA has been reported to be the most common odontogenic tumour in dogs.17,18
In the present study, there was no correlation between breed and tumour type except for MM and AA. Cocker spaniels, Poodles and German shepherds exhibited a predilection for the first type, while Boxers and Golden Retrievers for the second. Sample size of this study could account for the finding. Cocker spaniels and Poodles are frequently affected by MM.7,19 There are contradictory reports regarding the predilection for German shepherds.19 Finally, AA is usually encountered in large breeds.20
Malignant melanoma and SCC were most commonly found in older dogs of our study (mean age 11.95 and 7.8 years respectively).5,17 Sex predilection for fibrosarcoma, POF, AA, and oral papilloma in this study is consistent with the literature.17,21,22
Conclusions concerning the location of appearance could only be drawn for the AA and the MM due to the small number of other types. Both types were mostly found on the mandibular gingiva.23 Overall, the majority of neoplasms were located in the caudal mandible. As far as the MM is concerned, tumours were located caudally in 4 cases, 3 of which recurred locally, while the 4th exhibited major complications (extensive wound hehiscence). Caudal tumour location is a poor prognostic indicator for most oral tumours.23,24 This may be because of the challenges associated with more caudal surgeries, including the inability to achieve clean surgical margins, or to the late stage in which these tumors are often detected by the owners. This finding might play part in the median disease free interval (DFI). Size of the neoplasm was associated with the outcome. Primary tumour size has been found to be extremely prognostic in canine oral MM.25 In the present study, 90.9% of the MMs was defined as T2+T3 size (according to WHO) and only 9% of the dogs survived more than 365 days. Additionally, in the present study, poor outcome was associated with tumour size when all types were combined (rs=0.481, p=0.000). The fact has also been stated in human maxillofacial surgery,26 while co-estimation of tumour thickness and depth of invasion are also considered important prognostic indexes in some types of oral neoplasms.27,28
Various degree of bone invasion of the jaws was observed in the majority of the malignant neoplasms (63.88%) and AAs in our study. Osteolysis has been reported in 60-80% of all neoplasms combined.29
In the current study, dogs with thoracic metastasis at the time of admission were excluded as inoperable with the exception of 2 animals that underwent palliative surgery. According to other studies, MM tends to metastasise in 80%,30,31 fibrosarcoma in 30% and non-tonsilar SCC in 20% of the cases respectively.29 According to Todoroff and Brodey (1979),32 necropsy findings indicated at least 13% false-negative thoracic radiographs in dogs with MM and that 81% of MMs, 77% of tonsillar SCCs, and 35% of fibrosarcomas had already metastasised to or beyond regional LNs. Ki-67 proliferation index was associated with LN metastasis for gingival SCC.33 Staining outcome for AgNORs and Ki-67, which has been associated with survival in canine soft tissue sarcomas outside of the oral cavity, could potentially provide additional information in estimating survival in dogs with oral sarcomas.34 Benign neoplasms do not tend to metastasise.22 Lymph node size has been shown to have poor sensitivity and specificity as a predictor of metastasis (70 and 51%,respectively). In the present study, LN infiltration was significantly associated with poor outcome. Therefore, cytologic or histologic examination of regional LNs should be performed for the most accurate staging regardless of size.35
Histopathological examination provides the cornerstone for establishing a definite diagnosis.36,37 The tumour type will define the treatment plan and prognosis. Lack of proper staging, represents a limitation of the present study. Mandibulectomy or maxilectomy is the treatment of choice in all cases of malignancy and AA. The extent of bone removal is dictated by the size of the lesion and bone involvement. 20 Clean histological margins should always be assessed. Given the retrospective nature of this study it was not possible to further characterise the histological margins as close or narrow, which may have provided additional prognostic information and also explained the relatively high rate of local recurrence despite macroscopically clean margins.
Excision of bone and soft tissues is indispensable for long-term survival for dogs with MM,38 and AA.39 Despite its benign behaviour, AA tends to recur after conservative excision.39 Radiation therapy is indicated for AAs which are not curable with surgery alone.40 However, even with a narrow-margin excision, AA surveillance would be an appropriate management recommendation as it has never been shown to metastasise in dogs.61 Adjuvant CSPG4-antigen electrovaccination42 and huTyr DNA vaccine43 have been used in dogs as adjunctive treatment for MM. Cisplatin combined with piroxicam has an antitumour activity against MM and SCC.44 Response rate of SCC when treated with adjuvant piroxicam alone was similar to that reported for other cytotoxic treatments.45 Radiation protocols for SCC have been also applied in veterinary medicine.46 Fibrosarcoma carries a guarded prognosis as it exhibits aggressive behaviour with 24-59% recurrence rate and metastasises in 30% of the cases.23,29,47,48 Mastinib and imatinib possibly combined with doxorubicin have been tested in vitro and could be applied as an adjuvant therapy.49 Additionally, combination of surgery and radiation can prolong median survival in dogs with fibrosarcoma. 47 The standard of care for oral sarcoma is still a combination of surgery and curative radiation, when possible. However, if surgery is not possible, radiation therapy used alone could be attempted. For older dogs or dogs with large tumours, a course of palliative radiation is useful to reduce the tumour burden and improve the quality of life for several months.46 In the current study, fibrosarcomas had the highest rate of recurrence. Marginal excision with osteoplasty is usually utilised in POF and excisional biopsy with or without concomitant administration of azithromycin (10 mg kg-1 SID for 10 days) is used in cases of oral papillomatosis.
Conclusions
Survival information was acquired by physical examination or contacting owners and necropsies were not routinely performed. Therefore, events of metastasis might have been missed. Moreover, local recurrence might have been underreported, because most of the owners do not tend to inspect their pet’s oral cavity. However, based on the results of the present and previously reported studies, curative-intent surgery alone is important in order to improve DFI and the cause specific survival.50
Conflict of interest
Authors declare no conflict of interest.
References
1. Brodey RS, Kelly DF. Thyroid neoplasms in the dog. A clinicopathologic study of fifty-seven cases. Cancer 1968, 22:406-416.
2. Birchard SJ, Roesel OF. Neoplasia of the thyroid gland in the dog: a retrospective study of 16 cases. J Am Anim Hosp Assoc 1981, 17:369- 372.
3. Hararι J, Patterson JS, Rosenthal RC. Clinical and pathologic features of thyroid tumors in 26 dogs. J Am Vet Med Assoc 1986, 188:1160-1164.
4. Wucherer KL, Wilke V. Thyroid Cancer in Dogs: An Update Based on 638 Cases (1995-2005). J Am Anim Hosp Assoc 2010, 46:249-254.
5. Loar AS. Canine thyroid tumors. In Current Veterinary Therapy IX. Kirk RW (ed). WB Saunders: Philadelphia, 1986, pp.1033-1039.
6. Barber LG. Thyroid tumors in dogs and cats. Vet Clin North Am Small Anim Pract 2007, 37:755-773. 7. Klein MK, Powers BE, Withrow SJ, Curtis CR, Straw RC, Ogilvie GK, Dickinson KL, Cooper MF, Baier M. Treatment of thyroid carcinoma in dogs by surgical resection alone: 20 cases (1981-1989). J Am Vet Med Assoc 1995, 206:1007-1009.
8. Théon AP, Marks SL, Feldman ES, Griffey S. Prognostic factors and patterns of treatment failure in dogs with unresectable differentiated thyroid carcinomas treated with megavoltage irradiation. J Am Vet Med Assoc 2000, 216:1775-1779.
9. Worth AJ, Zuber RM, Hocking M. Radioiodide (131) I therapy for the treatment of canine thyroid carcinoma. Aust Vet J 2005, 83:208-14.
10. Carver JR, Kapatkin A, Patnaik AK. A Comparison of medullary thyroid carcinoma and thyroid adenocarcinoma in dogs: A retrospective study of 38 cases. Vet Surg 1995, 24:315-319.
11. Leav I, Schiller AL, Rijnberk A, Legg MA, Der Kinderen PJ. Adenomas and Carcinomas of the Canine and Feline Thyroid. Am J Pathol 1976, 83:61-122.
12. Hayes HM, Fraumeni JF. Canine thyroid neoplasms: epidemiologic features. J Natl Cancer Inst 1975, 55:931-934.
13. Turrel JM, McEntee MC, Bruke BP, Page RL. Sodium iodide I 131 treatment of dogs with nonresectable thyroid tumors: 39 cases (1990- 2003). J Am Vet Med Assoc 2006, 229:542-548.
14. Fineman LS, Hamilton TA, Gortari A, Bonney P. Cisplatin chemotherapy for treatment of thyroid carcinoma in dogs: 13 cases. J Am Anim Hosp Assoc 1998, 34:109-112.
15. Benjamin SA, Saunders WJ, Angleton GM, Lee AC. Radiation carcinogenesis in dogs irradiated during prenatal and postnatal development. J Radiat Res 1991, 32:86-103.
16. Verschueren CP, Rutteman GR, Vos JH, Van Dijk JE, De Bruin TW. Thyrotrophin receptors in normal and neoplastic (primary and metastatic) canine thyroid tissue. J Endocrinol 1992, 132:461-468.
17. Benjamin SA, Stephens LC, Hamilton BF, Saunders WJ, Lee AC, Angleton GM, Mallinckrodt CH. Associations between lymphocytic thyroiditis, hypothyroidism, and thyroid neoplasia in beagles. Vet Pathol 1996, 33:486-494.
18. Suarez HG, du Villard JA, Severino M, Caillou B, Schlumberger M, Tubiana M et al. Presence of mutations in all three ras genes in human thyroid tumors. Oncogene 1990, 5:565-570.
19. Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer 2006, 6:292-306.
20. Verschueren CP. Flow-Cytometric DNA Ploidy analysis in primary and metastatic canine thyroid carcinomas. Anticancer Res 1991, 11:1755- 1761.
21. Bertazzolo W, Giudice C, Dell’Orco M, Caniatti M. Paratracheal cervical mass in a dog. Vet Clin Pathol 2003, 32:209-212.
22. Almes KM, Heaney AM, Andrews JA. Intracardiac ectopic thyroid carcinosarcoma in a dog. Vet Pathol 2008, 45:500-504.
23. Lantz GC, Salisbury SK. Surgical excision of ectopic thyroid carcinoma involving the base of the tongue in dogs: three cases (1980-1987). J Am Vet Med Assoc 1989, 195:1606-1608.
24. Broome MR, Peterson ME, Walker JR. Clinical features and treatment outcomes of 41 dogs with sublingual ectopic thyroid neoplasia. J Vet Intern Med 2014, 28:1560-1568.
25. Liptak JM, Kamstock DA, Dernell WS, Ehrhart EJ, Rizzo SA, Withrow SJ. Cranial mediastinal carcinomas in nine dogs. Vet Comp Oncol 2008, 6:19-30.
26. Constantino-Casas F, Rodriguez-Martinez HA, Gutierrez Diaz- Ceballos ME. A case report and review: the gross, histological and immunohistochemical characteristics of a carcinoma of ectopic thyroid in a dog. Br Vet J 1996, 152:669-672.
27. Kiupel M, Capen C, Miller M, Smedley R. Histological classification of the endocrine system of domestic animals Washington. Armed Forces Institute of Pathology, 2008, pp.64-68.
28. Patnaik AK, Lieberman PH. Gross, histologic, cytochemical and immunocytochemical study of medullary carcinoma in sixteen dogs. Vet Pathol 1991, 28:223-233.
29. Susaneck SJ. Thyroid tumors in the dog. Compend Cont Educ Pract Vet 1983, 5:35-38.
30. Taeymans O, Peremans K, Saunders JH. Thyroid imaging in the dog: current status and future directions. J Vet Intern Med 2007, 21:673-684.
31. Simpson AC, McCown JL. Systemic hypertension in a dog with a functional thyroid gland adenocarcinoma. J Am Vet Med Assoc 2009, 235:1474-1479.
32. Seguin B, Brownlee L, Walsh PJ. Endocrine system. In Veterinary Surgical Oncology. Kudnig ST, Sequin B(eds). Wiley-Blackwell: Chichester, 2012, pp.405-441.
33. Wisner ER, Nyland TG. Ultrasonography of the thyroid and parathyroid glands. Vet Clin North Am Small Anim Pract 1998, 28:973-991.
34. Weber AL, Randolph G, Aksoy FG. The thyroid and parathyroid glands. CT and MR imaging and correlation with pathology and clinical findings. Radiol Clin North Am 2000, 38:1105-1129.
35. Feldman N. Canine thyroid tumors and hyperthyroidism. In Canine and Feline Endocrinology and Reproduction. Feldman N (ed). 3rd ed. Saunders: Philadelphia, 2003, pp.219-249.
36. Taeymans O, Penninck DG, Peters RM. Comparison between clinical, ultrasound, CT, MRI, and pathology findings in dogs presented for suspected thyroid carcinoma. Vet Radiol Ultrasound 2013, 54:61-70.
37. Taeymans O, Schwarz T, Duchateau L, Barberet V, Gielen I, Haskins M, van Bree H, Saunders J. Computed tomographic features of the normal canine thyroid gland. Vet Radiol Ultrasound 2008, 49:13-19.
38. Liptak JM. Canine thyroid carcinoma. Clin Tech Small Anim Pract 2007, 22:75-78.
39. Flanders JA. Surgical therapy of the thyroid. Vet Clin North Am Small Anim Pract 1994 , 24:607-621.
40. Kent MS, Griffey SM, Verstraete FJ, Naydan D, Madewell BR. Computerassisted image analysis of neovascularization in thyroid neoplasms from dogs. Am J Vet Res 2002, 63:363-369.
41. Sullivan M, Cox F, Pead MJ, McNell P. Thyroid tumours in the dog. J Small Anim Pract 1987, 28:505-512.
42. Owen LN. TNM Classification of Tumours in Domestic Animals. 1st ed. World Health Organization: Geneva, 1980, pp.51-53.
43. Lunn KF, Page RL. Tumors of the endocrine system. In Withrow and MacEwen’s Small Animal Clinical Oncology. Withrow SJ, Vail DM, Page RL (eds) 5th ed. Elsevier: St Louis, 2013, pp.504-531.
44. Michail S. Comparative Anatomy of the Domestic Animals. 2nd edn. D Kyriakidis: Thessaloniki, 2015.
45. Hullinger RL. The endocrine system. In Miller’s Anatomy of the Dog. Evans HE (ed). 3rd ed. WB Saunders Co: Philadelphia, 1993, pp.559-585.
46. Radlinsky MG. Thyroid surgery in dogs and cats. Vet Clin Small Anim 2007, 37:789-798.
47. Fukui S, Endo Y, Hirayama K, Taniyama H, Kadosawa T. Identification and preservation of the parathyroid gland during total thyroidectomy in dogs with bilateral thyroid carcinma: a report of six cases. J Vet Med Sci 2015, 77:747-751.
48. Kornegay JN. Hypocalcemia in dogs. Compend Cont Educ Pract Vet 1982, 4:103-110.
49. Tuohy JL, Worley DR Withrow SJ. Outcome following simultaneous bilateral thyroid lobectomy for treatment of thyroid gland carcinoma in dogs: 15 cases (1994–2010). J Am Vet Med Assoc 2012, 241:95-103.
50. Pack L, Roberts R, Dawson SD, Dookwah HD. Definitive radiation therapy for infiltrative thyroid carcinoma in dogs. Vet Radiol Ultrasound 2001, 42:471-474.
51. Jeglum KA, Whereat A. Chemotherapy of canine thyroid carcinoma. Compend Cont Educ Pract Vet 1983, 5:96-98.
52. Nadeau ME, Kitchell BE. Evaluation of the use of chemotherapy and other prognostic variables for surgically excised canine thyroid carcinoma with and without metastasis. Can Vet J 2011, 52:994-998.
Corresponding author:
Maria I. Kouki
119 Goura st.
18546 Piraeus
e-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.



