> Abstract
Companion animals maintain their body temperature through thermoregulatory mechanisms located in the hypothalamus that stimulate the production or reduction of heat accordingly in the event of a sudden fall or increase in environmental temperature. Hypothermia occurs when the body temperature falls below 37.5οC. Young, aged, malnourished and small animals are more susceptible. Hypothermia can be caused by exposure to low ambient temperatures or through the disruption of thermoregulatory mechanisms due to various factors including surgical procedures, trauma, systemic disorders, drug administration etc. A drop in temperature results in a lower basal metabolic rate and abnormal function of the cardiovascular, respiratory, urinary and central nervous systems, as well as acid-base balance and haemostasis. Bradycardia, hypotension, bradypnoea, pulmonary hypoventilation and deterioration of consciousness, shivering, hypovolaemia, electrolyte disorders, hyperglycaemia, as well as respiratory and metabolic acidosis are exhibited. Treatment includes rewarming therapy and stabilization via oxygen and fluid administration. The rewarming techniques may be external or internal. External passive techniques include wrapping the animal in blankets and heating its environment. Active external rewarming entails placing a heat source around the animal’s body, such as warm iv solution bags and heating pads, whereas active internal rewarming involves intravenous therapy of warm fluids and more invasive techniques such as abdominal or pleural lavage with warmed solutions.
> Thermoregulatory system
Cats and dogs are homeothermic animals since their normal body temperature remains stable regardless of ambient temperature. However, this applies only within a certain range of environmental temperatures, beyond which mechanisms of heat production or loss are activated accordingly in order to preserve a steady core temperature.1
Body temperature is classified as core temperature, which is the temperature of internal organs with extensive blood supply (the brain, thoracic and abdominal cavity organs), and as peripheral temperature which is measured near the body surface.2,3 The latter may vary according to physical activity, ambient temperature and the presence of a heat source in the same location.2 This can lead to a considerable difference between peripheral temperature and that of internal organs.2,3 Hence, it is important to measure core temperature accurately.2
Τhe main thermoregulatory centre of the body is the hypothalamus. Specifically, the anterior segment of the hypothalamus houses central thermoreceptors. The temperature of blood circulating through the hypothalamus is an immediate trigger for these receptors. Stimuli from peripheral thermoreceptors in the integument and deeper body tissues, such as the spinal cord, abdominal organs and large blood vessels of the thoracic and abdominal cavity, reach the hypothalamus through the spinal cord.1-4 Posterior hypothalamus stimulation activates mechanisms of heat production or reduction.1,3
Normally, the production of heat in the body stems from the basal metabolic rate, especially in the most metabolically active organs such as the heart, brain and liver, and from voluntary physical activity (exercise). 1,3 When the body is exposed to cold, the basal metabolic rate increases and vasoconstriction of skin arterioles and piloerection are incited to help preserve heat.1-6 At the same time, adrenaline, noradrenaline, thyroid hormones and glucocorticoids are excreted due to their heat-producing effect.1,5 The normal physical response to cold is activated by a drop in core temperature of a mere 0.25 οC.3,7 It is worthy of note that in the paw pads of carnivores, arterioles become dilated in close anatomic proximity to venules, resulting in the warming of blood through the periphery of the limbs before returning to the body core.8 At the same time, physiological responses of the animal to cold conditions are stimulated, such as curling up in order to reduce body surface exposure to cold and huddling, in the case of those living in groups.1,2,5,6 When ambient temperature is low enough, heat is produced mostly through muscle tremors (shivering), which are involuntary, rhythmical and high frequency contractions of skeletal muscles.1-6,9
Conversely, when ambient temperatures are elevated, metabolic rate and muscle tone are initially reduced, peripheral vasodilation occurs and excretion of the aforementioned thermogenic hormones diminishes, thereby decreasing heat production.1 Loss of body heat generally occurs with the evaporation of Η2Ο, mostly from the respiratory tract, intensifying with open mouth tachypnoea (panting) in animal species that cannot produce sweat.1,2,5 With tachypnoea, respiratory dead space ventilation increases to facilitate evaporation of water from airway mucosae which results in heat emission and consequent drop in body temperature. This increase in respiratory frequency usually coincides with intense salivation, leading to additional heat loss. Furthermore, the extension and relaxation of the tongue further intensifies the evaporation of water due to the wider available surface.1 Heat is secondarily emitted through radiation from the skin towards cold surfaces without physical contact as well as via contact of the skin with colder objects (conduction) through the layers of air surrounding the body (convection), faeces, urine etc.1-5
> Hypothermia
Hypothermia is the reduction of core temperature below the normal range for each animal species, (<37,5 οC: dog, <37,8 οC: cat) due to an inefficiency of the body to preserve its thermal homeostasis.2,6,7,10,11
- Epidemiology/Frequency of occurrence
The age and body size of the animal play a major role in the development of hypothermia. The young, aged, malnourished or small animals are most vulnerable, with cats being the most easily affected. Very young animals or those with poor body condition have a low basal metabolic rate, an only partly developed mechanism of shivering and, as in small animals, a large surface to body weight ratio. 3,4,6,7,10,12-15 Aged pets have a low basal metabolic rate and reduced muscle tone.15 Moreover, given that the first energy molecules expended are glucose molecules, the very young as well as weakened or aged animals are more vulnerable to hypothermia since they have low reserves of glycogen and reduced glyconeogenic potential.16
- Aetiology
Depending on the causing factor, hypothermia is classified as primary or accidental and secondary. 2,5,17 Primary hypothermia is caused by extended exposure to low environmental temperatures, such as during cold conditions, raining or cold water immersion, in which cases heat loss intensifies regardless of the fact that the thermoregulatory mechanisms are functioning normally.2,5,6,10,17,18 Secondary hypothermia is caused by any condition that reduces heat production or increases heat loss by disrupting the thermoregulatory system even in warm ambient conditions.2,5,17 In this case, it can be the result of trauma, burns, surgical procedures, drugs, mainly anaesthetics, and numerous conditions such as hypothyroidism, hypoadrenocorticism (Addison’s disease), suboptimal function of the hypothalamus, uraemia, circulatory shock, CNS disorders, various cardiomyopathies and nephropathies, malnutrition, hypoglycaemia and toxicoses.2,5,6,10,17,18
Among the most common causes of hypothermia in daily clinical practice are trauma, administration of anaesthesia, and various surgical procedures of which 60-90% display hypothermia.7,19,20 In one retrospective study of 275 cats, hypothermia developed as a perianaesthetic complication in 96.7% of cases.21 The development of hypothermia is attributed to several factors including the low temperature and relative humidity in the operating theatre, administration of cool intravenous fluids, inhalation of cool and dry anaesthetic gases during inhalational anaesthesia, as well as the preparation of the surgical site with antiseptic alcohol-based solutions which promote heat loss through evaporation. 3,13,14,22-25 The type of anaesthetic medication, as well as the anaesthetic system, also play a major role. Several of the medications administered as pre-anaesthetic or general anaesthesia induction agents, such as phenothiazines, morphine, propofol, thiopentone and inhalants, cause peripheral vasodilation leading to increased heat loss, suppressed hypothalamic function and muscle tremors, and reduced muscle tone.9,12,14,17,22,24,25 In addition, control of heat loss from the body through vasoconstriction and other mechanisms is activated in anaesthetized animals after a temperature reduction of 2.5-3οC which, under normal circumstances, is observed in temperature drops of 0.25 οC.7,20 This indicates that lower temperatures are required to elicit a response from the hypothalamic thermoregulatory centre in animals under anaesthesia.13,25 In non-rebreathing circuits (e.g. paediatric Jackson-Rees modification of the Ayre’s T-piece), the inhaled gases are warmed during the passage through the animal’s airways and at exhalation they are removed from the respiratory circuit to the environment, thus losing significant amounts of heat, whereas cooler gases are freshly inhaled.15 Another contributory parameter in the development of hypothermia concerns the type of surgical procedure. In cases of abdominal organ exposure to the environment during thoracotomy or laparotomy, the core temperature is significantly reduced due to convection resulting from the large contact surface with atmospheric air and because of water evaporation from the mucosae, conditions that can account for up to 50% of the total amount of heat lost during surgery.3,7,10,13,14,21-26 Orthopaedic surgeries also require extensive preparation of the surgical site, thereby increasing heat loss.21 Finally, the duration of anaesthesia is an equally important factor given that in prolonged surgical procedures, the effect of the above factors is extended as is the physical toll.4,10,13,15,21,26
- Physical examination and laboratory findings
Hypothermia has been sub-classified into three basic degrees of severity: mild hypothermia (37°C to 32°C) during which thermoregulatory responses continue to function normally; moderate hypothermia (32°C to 28°C) with gradual loss of thermoregulatory response, especially that of shivering; and severe hypothermia (<28°C) with full suppression of thermoregulation homeostasis and muscle rigidity (Table 1).2,5,6,13 Death occurs at temperatures of about 20°C.11
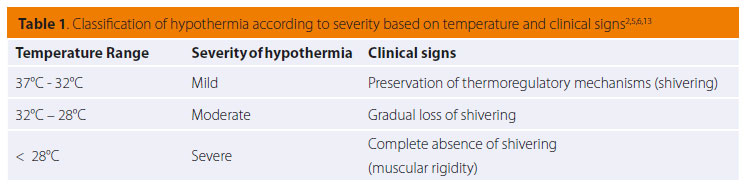
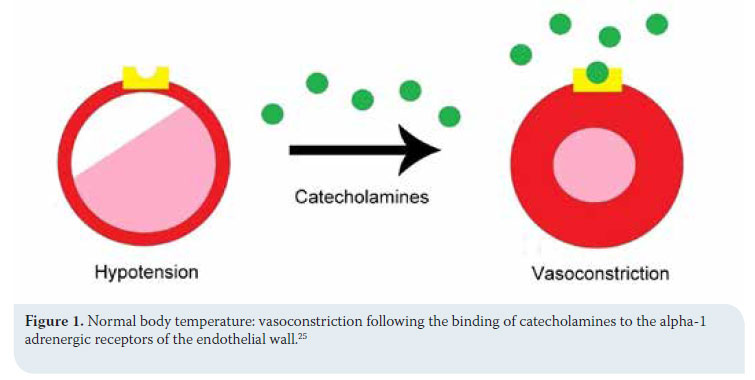
Clinical signs stem from most body systems. More specifically, regarding the cardiovascular system, tachycardia and peripheral vasoconstriction are initially observed with possible preservation or increase in arterial blood pressure due to the excretion of catecholamines (adrenalin/noradrenalin), as previously mentioned, in order to prevent heat loss.2,5,13,14 When hypothermia is prolonged, the α1 adrenergic receptors cease to respond to catecholamines and peripheral vasoconstriction and hypotension ensue (Figures 1 and 2).2,5,18,19,25 Τachycardia is followed by severe bradycardia, non-responsive to atropine.2,5,6,9,11,18 Arrhythmias, both atrial and ventricular, are common.2,5,9,13,18,25 The initial arrhythmia is atrial fibrillation, which can devolve into ventricular tachycardia and fibrillation.2 In fact, in a study during which hypothermia was experimentally caused in dogs, ventricular fibrillation was noted in 50% of cases with body temperatures below 23.5οC.18,25 Final stages include asystole and cardiac arrest.2,5,9,18
In the respiratory system, as hypothermia progresses, the reduction in metabolic rate and consequent decrease in produced CO2 contributes to lower numbers of breaths per minute as well as tidal volumes. At the same time, blunting of the airway protective reflexes (sneezing, coughing) generates an increased risk of aspiration.2,11 Severe hypothermia can possibly cause non-cardiogenic pulmonary oedema, while severe bradypnoea and pulmonary hypoventilation are commonly observed, ultimately leading to apnoea.2,5,11
As concerns the central nervous system, temperature reductions produce an altered level of consciousness, which can vary from depression to lethargy, as a result of reduced cerebral perfusion. At the same time, hyporeflexia is noted. Shivering is activated during mild hypothermia but ceases with persistent heat loss. During severe hypothermia, there is usually an absence of reflexes accompanied by mydriasis and finally coma, due to cerebral ischaemia and hypoxia.2,5,6,10,13
The most significant finding in the urinary system is diuresis, regardless of the degree of dehydration (cold diuresis), which causes significant hypovolaemia and hypotension.2,5,6,14 The initial peripheral vasoconstriction during the onset of hypothermia results in a relative increase in circulating blood volume followed by diuresis in order to reduce the former. 2,13 As body temperatures continue to fall, the response of the distal renal tubules to the effect of the anti-diuretic hormone is reduced and water reabsorption by the kidneys is limited, thus prolonging the loss of water and electrolytes such as phosphorus, potassium and magnesium.2,14,17 The reabsorption of glucose is also restricted which leads to glycosuria.6,14 Furthermore, hyperglycaemia is induced due to tissue resistance to insulin or reduced production of insulin from the pancreas, secondary to pancreatitis following reduced pancreatic perfusion. Hyperglycaemia is also caused by the secretion of catecholamines and glucocorticoids.2,5,6,17,18
In addition, hypothermia causes severe but reversible disruptions in coagulation, affecting both primary and secondary haemostasis.2,23 Other than thrombocytopenia, caused by the arrest of platelets in the liver and spleen or the suppression of the production and release of platelets by the bone marrow due to the cold, there is also a disruption in platelet function resulting in the delay of initial clot formation.2,6,13,14 However, increased coagulation and disseminated intravascular coagulation may occur rather than haemorrhagic diathesis.2,5,6 This could arise due to several factors such as cardiovascular suppression, disruption of blood microcirculation, release of thromboplastin from cool tissues, or haemoconcentration owing to hypovolaemia from «cold diuresis».2,5,13,17 Moreover, given that the enzymes of the coagulation cascade are heat-dependent, prothrombin time (PT) and partial thromboplastin time (PTT) could increase.2,5,11 It should be stressed, however, that the various conventional coagulation tests are usually performed at 37oC, giving laboratory results that do not coincide with physical examination findings in hypothermic patients.2,5,6,23,25
Regarding the effect of hypothermia on acid-base balance, hypothermia causes metabolic as well as respiratory acidosis.2,5,10 Metabolic acidosis is observed due to the reduced secretion of hydrogen ions (H+) by the kidneys and the increased production of lactic acid caused by muscle tremors during the initial stages of hypothermia. On the other hand, respiratory acidosis can be attributable to the reduced removal of produced carbon dioxide by the lungs due to bradypnoea and inefficient alveolar ventilation, notwithstanding the decreased production of carbon dioxide resulting from the lower metabolic rate in hypothermic conditions, as previously mentioned.2,11 Additionally, the solubility of CO2 is higher in colder blood, leading to elevations in its concentration.2
During surgical procedures, the drop in core temperature can cause considerable complications to anaesthetized animals, owing to the lower basal metabolic rate, which in fact reduces 10% for every 1οC of reduced temperature.11 Beyond the aforementioned effect on various organ systems, decreased metabolism and clearance of anaesthetic drugs by the liver is also noted due to its reduced perfusion. This results in increased anaesthetic potency or depth, prolonged duration of anaesthesia etc.2,3,7,11,13,21,22,25 Τhe most common drugs that can incur a delay in metabolic processing and detoxification, thereby increasing the risk of overdose, include propofol, midazolam, morphine, acepromazine, fentanyl, phenobarbital, and inhalational anaesthetics. 2,22,25 Additionally, the solubility of inhalatory anaesthetics in the blood increases with the drop in temperature, delaying their release from the body.22 The minimal alveolar concentration (MAC) of inhalatory anaesthetics (e.g. isoflurane for rats) is reduced by 5% for every 1°C of core temperature reduction. 27,28 The final result is prolonged recovery, as well as delayed healing or infection of the surgical site since hypothermia can also suppress the normal function of the immune system.2,3,11,13,19,21,22,24,25,29,30 Furthermore, during the recovery stage, the return of the thermoregulatory responses, especially shivering, increases the metabolic heat production at the expense of increased oxygen demands by up to 500%, which is not easily tolerated by a weakened animal and could cause hypoxia, mostly in patients with respiratory disorders.7,11,13,15,24,30 Nonetheless, according to a retrospective study of 275 cats, perianaesthetic hypothermia can rarely prove fatal.21
- Management
Treating hypothermia is based on general stabilization and rewarming prior to managing the primary cause in cases of underlying systemic disorders.2,10
• Stabilization and medical treatment
Initially, measures are taken to stabilise the cardiovascular and respiratory system. Specifically, airway patency is controlled and restored when necessary and Ο2, fluids and electrolytes are administered.2,6,17 Intravenous fluid therapy is used to control hypotension stemming from «cold diuresis» and to increase blood volume, and must be administered immediately. Crystalloid solutions, to which potassium is added when necessary, are administered intravenously after being warmed.2,6,17 In cases of severe hypotension, a specific amount of fluids can be given as a bolus. The temperature of solutions entering the body of the animal should not surpass 42oC.17 Furthermore, the administration of Lactated Ringer’s should be avoided in severely hypothermic animals, because the liver no longer possesses the ability to metabolize the lactate. The use of saline, or even the mixing of 1/3 dextrose with 2/3 of saline, is considered safer.6,17,18 Controlling the rhythm and amount of fluids administered can be more accurate by measuring central venous pressure and urine output.6,14,17 However, the use of catheter placement for the aforementioned measurements demands caution because of coagulation disorders that may be present in a hypothermic animal.6
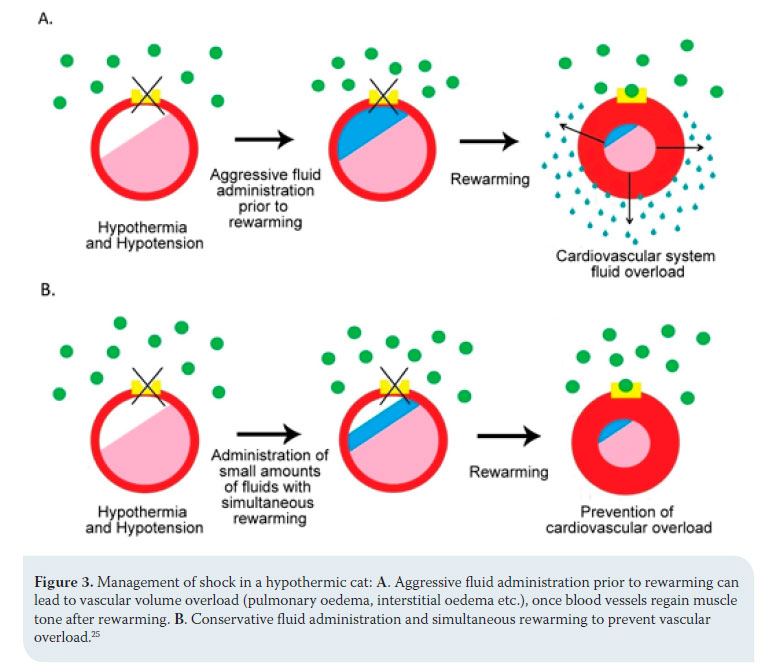
The order and intensity with which therapeutic measures are followed can be differentiated according to animal species. In dogs, the administration of fluids is aggressive and precedes rewarming, otherwise peripheral vasodilation caused by the latter will deteriorate hypotension and poor perfusion of tissues and reduce the speed of temperature restoration. 25 In cats, the risk of volume overload and pulmonary oedema is palpable once blood vessels regain their muscle tone after rewarming and activation of the adrenaline receptors.17,25 For this reason, the animal is given small amounts of fluid and aggressive rewarming is applied (Figure 3).25
Hypothermic animals should be handled with care since even the slightest stimulation can lead to cardiac arrhythmias; moreover, the hypothermic heart is unable to respond to medical treatment. As previously mentioned, bradycardia is unresponsive to atropine in low temperatures and rewarming alone is usually sufficient. In general, antiarrhythmic and vasoactive medications should not be administered until body temperature reaches 30-32oC as they will be ineffective.2,6,10,17,18 Moreover, repeated use of such drugs is not recommended because after rewarming the total doses could become toxic due to their additive effect.17,18
Given that hypothermia can limit the immune response, it is considered necessary to administer wide-spectrum antibiotics to prevent secondary opportunistic infections such as pneumonia, infection of the surgical site etc.2,14 Furthermore, since severe pain is experienced during rewarming, it is important to administer analgesics from the moment body temperature reaches 37οC.6,17,18 Τhe limbs, ears and tail may be particularly painful; this should be taken into consideration during handling of hypothermic animals. The ideal choice of medication is opioids. If renal function and tissue perfusion have been restored, non-steroidal anti-inflammatory drugs can also be administered.17 Bearing in mind that hepatic metabolism is reduced, any drugs should always be administered with care.2,15
• Rewarming
Hypothermic animals can sometimes be perceived as dead due to the fact that breathing and heart rate are not easily detected and pulses may not be palpable, especially in temperatures below 28oC.5,6,14,17,18 Poor conductivity of cold skin can render even an electrocardiogram difficult to interpret. 18 Consequently, such animals must not be considered as dead before rewarming.5,6,14,17 The exact measuring of core body temperature is possible through the thoracic oesophagus, though this is accomplished only in anaesthetized or comatose animals.2,4,20 Rectal temperature measurement is used more commonly since it is a practical and easier method; however, it usually differs 0.4οC from the actual core temperature.2,3,20 The rewarming method to be applied depends on the severity and duration of hypothermia, the animal’s state, and the initiating cause.6,10,17 There are three methods of rewarming: passive, external active and internal active rewarming. Independent of the technique, the temperature must increase by 1-2οC per hour. All efforts should cease at 37-37.5οC to avoid iatrogenic hyperthermia as after this point, normal thermoregulatory responses are activated.5,25,29
Passive rewarming is based on the animal’s innate heat-producing ability and aims to preserve it, limiting heat loss towards the environment. It is mostly recommended in cases of mild hypothermia not caused by underlying disorders, during which normal thermoregulatory responses are still functioning. 2,5,6,10,13,14,17,19 It includes warming the patient’s environment, drying the animal with a towel if wet, and wrapping it in dry, insulating blankets, such as fabric or reflective aluminium (Table 2).2,5,10,13,14,17,19,24 It is considered good practice to also cover the head and limbs and not only the trunk.11,17
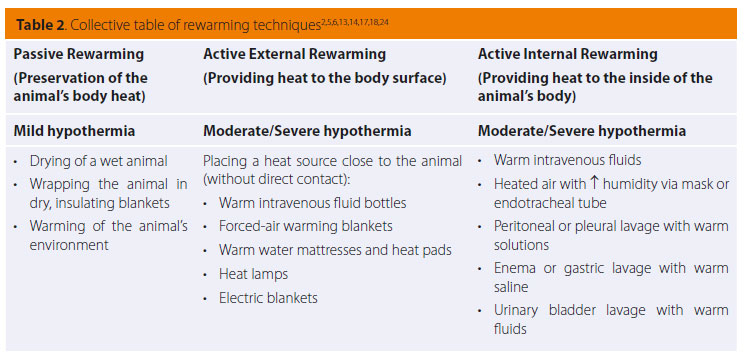
With active external rewarming, heat is applied to the surface of the animal with the aim of increasing the temperature of the surrounding air, thus reducing the difference in temperature between the skin surface and the environment, enabling heat loss via convection to be reduced.13,19 This method is indicated in cases of moderate to severe hypothermia caused by inefficient heat production in animals with hypothyroidism, hypoadrenocorticism, hypoglycaemia, cardiomyopathies and other disorders that decrease internal thermogenesis, as well as in cases of passive rewarming failure.2,5,6,17,18 According to this rewarming technique, a heat source such as hot intravenous fluid bottles, circulating warm water mattresses, heat pads, electric blankets and radiating heat lamps, are placed around the animal’s body (Table 2).2,5,6,13,14,17,18 Great care should be given to preventing skin burns. For that reason, the above heat-providing means should not come into direct contact with the animal, nor should their temperature exceed 45oC. Insulating materials such as blankets, towels or surgical draping can be placed between the animal’s body and the heat source; a frequent change in recumbency is also recommended to ensure that the same side of the animal is not exposed to heat for a prolonged period of time.5,10,13,14,17 A much safer and more effective technique includes providing hot air within special blankets wrapped around the animal (forced-air warming blankets).2,5,6,10,13,17,24 However, the use of external heat sources against the body, and especially the limbs, can present certain complications. Intense peripheral vasodilation caused by rewarming may lead to deterioration of hypotension and increase the metabolic demands of peripheral body tissues to which the cardiovascular system is unable to respond.2,5,13 This condition is called rewarming shock, and could possibly lead to sudden death.5 In addition, peripheral vasodilation results in relatively warmer core blood moving toward colder peripheral tissues and therefore cooling, while cooler blood from the periphery returns to the core, leading to deterioration of hypothermia (afterdrop), up to the point where the peripheral and core body temperatures fully balance.2,5,6,13,14 Consequently, it is preferable that this technique is limited to the trunk of the animal, or at least that thoracic rewarming should precede that of the limbs, so that the temperature and ability of the heart to perfuse the limbs is ensured. 2,5,6
The aim of active internal rewarming is the swift increase of core temperature through the administration of heat to inner tissues.13 This technique is indicated in animals with moderate to severe hypothermia and unsteady cardiovascular function, especially post arrest, and in cases of failure of the previous techniques.2,6,17,18 Examples of this method include the administration of warmed intravenous fluids, peritoneal or thoracic lavage with warm solutions, gastric lavage or hot water enemas, urinary bladder lavage with warm fluids and administration of heated air with increased humidity via oxygen mask or endotracheal intubation (Table 2).2,5,13,14,17,24,29 Warm crystalloid solutions that are intravenously administered during the stabilization process are a relatively ineffective means of rewarming because of the wide difference between the volume of fluids administered and the animal’s body mass, but also due to the fact that they quickly cool down by the time they enter the animal’s body. It would have been more effective if large fluid volumes were administered at a rapid rate, but this could incur an increase of risk.5,17 Pleural or peritoneal lavage is sufficiently effective, considering that this allows for immediate rewarming of basic metabolic organs such as the liver or the heart. In these techniques, isotonic crystalloid solutions are used, heated to about 42οC (40-45 οC).2,5,6,13,14 However, these are invasive procedures and should be avoided in cases of suspected trauma or recent surgery in these cavities, as well as in animals with haemostatic disorders due to hypothermia. 2,17 Heating the inhaled air or oxygen up to a temperature of 40-45 οC using a heater and humidifier between the oxygen tubing and the endotracheal tube/mask, or by placing a bottle of warm water on the tubing, is a non-invasive method which permits the control of heat loss from the respiratory tract.2,5,6,10,13,17,18 Finally, colonic, gastric and urinary bladder lavage with warm saline are less effective techniques with several complications, including electrolyte disorders, the risk of vomiting and aspiration, and the inability to measure the exact temperature through the rectum and esophagus since it will be seen to be increased.2,5,6,14,17
- Prevention
Preventing hypothermia prior to and during anaesthesia is possible and can be accomplished with systematic control of body temperature from the oesophagus by checking the temperature and relative humidity of the operating theatre, heating of fluids and gases prior to use, and by placing heat pads/sources or insulating blankets underneath or around the animal. Hypothermia can also be prevented by reducing surgical time and using rebreathing anaesthetic systems in which the warm and humid exhaled gases re-enter the respiratory tract after passing through soda lime (for which it should be noted that during binding with carbon dioxide there is a chemical reaction producing heat), thus preserving body temperature more effectively. 4,11,13,15,19,20,22,24,30 Furthermore, it is useful to insulate or maintain warmth for all parts of the animal’s body which are not being operated on, such as the limbs and tail, given that these body parts have a higher body surface to body weight ratio and therefore their heat loss is swift and severe.11,25 Indeed, in one study of 32 dogs, warming of the limbs proved to be more effective than that of the trunk in the prevention of hypothermia perioperatively because the limbs possess an extensive capillary network compared to the trunk; consequently, after anaesthesia-induced vasodilation, heat loss from the limbs is more pronounced. For the same reason, providing heat through the limbs and toward the body core is easier.7 Finally, another study of eight cats concluded that in combination with the aforementioned methods, the use of forced-air warming blankets during anaesthesia reduces heat loss via an increase in microenvironmental temperature surrounding the skin surface, and can be very useful in preventing hypothermia. However, it is difficult to apply in animals undergoing surgery, with the exception of cervical or cranial procedures.20
> References
1. Smokovitis A. Thermoregulation. Ιn: Physiology. 5th edn. Kyriakidis Bros-Editions S.A: Thessaloniki, 2007, pp. 870-892.
2. Todd JM. Hypothermia. In: Small animal critical care medicine. Silverstein DC, Hopper K (ed). 2nd edn. Elsevier: Canada, 2014, pp. 789-795.
3. Clark-Price S. Hypothermia in small animal patients. Vet Clin Small Anim 2015, 45(5): 983-994.
4. Evans AT. Anesthetic emergencies and accidents. In: Lumb and Jone’s veterinary anesthesia. Thurmon JC, Tranquilli WJ, Benson GJ (ed). 3rd edn. Williams and Wilkins: 1996, pp. 849-860.
5. Waddell LS, Boller EM. Environmental emergencies. In: Feline emergency and critical care medicine. Drobatz KJ, Costello MF (ed).1st edn. Willey-Blackwell: Iowa, USA, 2010, pp. 601-618.
6. Macintire DK, Drobatz KJ, Haskins SC, Saxon WD. Miscelaneous emergencies. In: Manual of small animal emergency and critical care medicine. Troy DB (ed). 1st edn. Lippincott Williams and Wilkins: 2005, pp. 402-414.
7. Cabell LW, Perkowski SZ, Gregor T, Smith GK. The effects of active peripheral skin warming on perioperative hypothermia in dogs. Vet Surg 1997, 26: 79-85.
8. Ninomyia Η, Akiyama Ε, Simazaki K, Oguri A, Jitsumoto M, Fukuyama T. Functional anatomy of the footpad vasculature of dogs: scanning electron microscopy of vascular corrosion casts. Vet Dermatol 2011, 22(6): 475-481.
9. Thurmon JC, Tranquilli WJ, Benson GJ. Injectable anesthetics. In: Lumb and Jone’s veterinary anesthesia. Thurmon JC, Tranquilli WJ, Benson GJ (ed). 3rd edn. Williams and Wilkins: 1996, pp. 210-240.
10. Jasani S. Hypothermia. In: Saunders solution in veterinary practice small animal emergency medicine. Nind F (ed). 1st edn. Saunders-Elsevier: 2011, pp. 74-75.
11. Dugdale A. Hypothermia: Consequences and prevention. In: Veterinary anaesthesia -principles to practice. Dugdale A (ed). ....1st edn. Willey-Blackwell: Oxford, United Kingdom, 2010, pp. 179-181.
12. Dugdale A. Monitoring animals under general anaesthesia. In: Veterinary anaesthesia principles to practice. Dugdale A (ed). 1st edn. Willey-Blackwell: Oxford UK, 2010, pp. 156-174.
13. Armstrong SR, Roberts BK, Aronson M. Perioperative hypothermia. J Vet Emerg Crit Care 2005, 15: 32-37.
14. Ahn AH. Approach to the hypothermic patient. In: Kirk’s current veterinary therapy XII Small animal practice. Bonagura JD, Kirk RW (ed). 12th edn. W.B Saunders Company: Philadelphia, 1995, pp. 157-161.
15. Kazakos G, Savvas I, Anagnostou Τ. Anaesthesia and intensive care. University lectures. School of Veterinary Medicine, Aristotle University of Thessaloniki, Thessaloniki, 2014.
16. Dugdale A. Neonates /paediatrics. In: Veterinary anaesthesia principles to practice. Dugdale A (ed). 1st edn. Willey-Blackwell: Oxford UK, 2010, pp. 312-314.
17. Mathews KΑ. Accidental hypothermia. In: Emergency critical care. Mathews KΑ (ed). 1st edn. Lifelearn: Canada, 2006, pp. 291-296.
18. Paitaki ΧG, Kazakos G. Accidental hypothermia and its treatment. 6th Hellenic Small Animal Veterinary Congress, 16-18 March 2001, Athens, Proceedings, pp. 520-527.
19. Potter J, Murrell J, MacFarlane P. Comparison of two passive warming devices for prevention of perioperative hypothermia in dogs. J Small Anim Pract 2015, 56: 560-565.
20. Machon RG, Raffe MR, Robinson EP. Warming with a forced air warming blanket minimizes anesthetic-induced hypothermia in cats. Vet Surg 1999, 28: 301-310.
21. Redondo JI, Suesta P, Gil L, Soler G, Serra I, Soler C. Retrospective study of the prevalence of postanaesthetic hypothermia in cats. Vet Rec 2012, 170: 206-209.
22. Pottie RG, Dart CM, Perkins NR, Hodgson DR. Effect of hypothermia on recovery from general anaesthesia in the dog. Aust Vet J 2007, 85: 158- 162.
23. Hackner SG, Rousseau A. Bleeding disorders. In: Small animal critical care medicine. Silverstein DC, Hopper K (ed). 2nd edn. Elsevier: Canada, 2014, pp. 554-567.
24. Hammond R. Anaesthesia and sedation of the critical patient. In: BSAVA manual of canine and feline emergency and critical care. King LG, Boag A (ed). 2nd edn. BSAVA: Gloucester, 2007, pp. 309-319.
25. Oncken AK, Kirby R, Rudloff E. Hypothermia in critically ill dogs and cats. Compend Contin Educ Small Anim Pract 2001, 23: 506-520.
26. Guillaumin J, Adin CA. Postthoracotomy management. In: Small animal critical care medicine. Silverstein DC, Hopper K (ed). 2nd edn. Elsevier: Canada, 2014, pp. 703-707.
27. Aranake A, Mashour GA, Avidan MS. Minimum alveolar concentration: ongoing relevance and clinical utility. Anaesthesia 2013, 68: 512–522.
28. Vitez TS, White PF, Eger EI 2nd. Effects of hypothermia on halothane MAC and isoflurane MAC in the rat. Anesthesiology 1974, 41: 80–81.
29. Mazzaferro EM. Perioperative evaluation of the critically ill patient. In: Small animal critical care medicine. Silverstein DC, Hopper K (ed). 2nd edn. Elsevier: Canada, 2014, pp. 691-694.
30. Egger C. Anaesthetic complications, accidents and emergencies. In: BSAVA Manual of canine and feline anaesthesia and analgesia. Seymour C, Duke-Novakovski T (ed). 2nd edn. BSAVA: Gloucester, 2007, pp. 310-332.



