> Abstract
Skin grafts are comprised of the epidermis and dermis. They are harvested from the donor site and transported to the recipient site of the same animal where they undergo adhesion, osmotic fl ow of plasma into the graft (plasmatic imbibition), vascular anastomosis and revascularization in order to be accepted by the recipient site. Full- thickness mesh skin grafts are most commonly used in the veterinary clinical setting in both dogs and cats for the reconstruction of skin defects located mainly on the limbs, where other reconstruction methods are not available. Grafts are placed on skin defects with healthy granulation tissue or on surgical wounds with adequate blood perfusion. Survival of feline grafts is far superior to that of canine grafts.
> Introduction
Skin grafts or free-skin grafts are slices of epidermis and dermis which are harvested by excision from an area of the trunk (donor site) and transported to a different location of the body (recipient site).1-7 Skin grafts must be differentiated from cutaneous fl aps and free cutaneous flaps. A cutaneous fl ap is composed of the epidermis and subcutaneous tissue with a base or pedicle through which it receives its vascular supply, and it can be transported from one site of the body to another. The free cutaneous fl ap is a type of flap where the cutaneous tissue is completely detached from its area of origin. It possesses an artery and vein and upon its placement on a different site, the blood vessels are reconstructed with microsurgical techniques and the artery and vein are reconnected with the corresponding blood vessels of the recipient site.3,8 In contrast to cutaneous fl aps, skin grafts are completely detached from the recipient site and have no vascular supply. Consequently, when placed on the recipient site, the viability of the graft depends entirely on the absorption of tissue exudate and the development of a new vascular supply.1,3,4,6,9 Cutaneous fl aps are recommended for areas with poor tissue perfusion, for burns, areas with constant movement (such as the axillae, elbows, tarsal or carpal areas) and for areas where increased tension is applied (such as the elbow, tarsal and carpal joint).1,3,6,7 However, when the option of a cutaneous fl ap is not available due to insuffi cient loose adjacent skin (as in the case of wounds on the lower limbs), a skin graft is the preferred solution.3,7,10-14 Grafts can also be used for the reconstruction of extensive trunk skin defects. The combination of flaps and grafts for the reconstruction of various defects is not uncommon.3,6,9,12
The aim of the present review is to describe the indications for skin graft placement in both dogs and cats, as well as the surgical techniques and postoperative complications. Furthermore, the pathophysiological mechanisms behind the process of graft-taking from the recipient site are investigated, as well as the causes of graft failure.
> Classification of grafts
Skin grafts can be classifi ed according to the relation between donor and recipient and the thickness of the epidermis and dermis from which the size and shape of the skin graft can be derived, in direct correlation to the defect of the recipient bed.1,3,9
In terms of the association between donor and recipient, skin grafts can be classifi ed as follows:
- Autografts or autogenous grafts: the donor and recipient site belong to the same animal.
- Allografts: the donor and recipient site belong to animals with a diff erent genotype, but still from the same species.
- Xenografts or heterografts: the donor and recipient site belong to animals from diff erent species.
- Isografts: grafts of tissue between monozygotic twins.
In veterinary practice, skin autografts are mostly used to achieve complete immunological compatibility between the harvested skin tissue and the recipient site. Based on the thickness of the harvested skin graft, it can be classifi ed as a full- thickness skin graft that includes the epidermis and the entire thickness of the dermis, or split-thickness (partial thickness) skin graft that comprises the epidermis and part of the dermis with variable thickness. Depending on the thickness of the harvested dermis included in the graft, split-thickness grafts can be further categorized as thin, intermediate, or thick.1,3,5,6,11,12
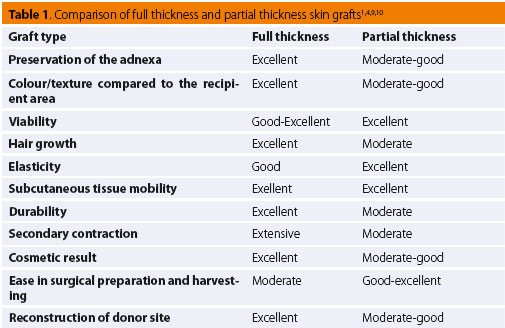
More specifi cally, full-thickness grafts are considered to be the most appropriate type of graft in small animal surgery.1-3,5,6,7,13,14 Such grafts can be used in the reconstruction of lower limb skin defects in dogs and cats.7,13,14 They are strong and capable of withstanding far less than ideal conditions during excision and relocation to the recipient site. All of the skin adnexa are preserved (such as hair follicles, apocrine and sebaceous glands), which are necessary for the normal function and cosmetic result of the graft (colour, texture, elasticity).5,7,10,13-15 Their range of motion and fl exibility are excellent when transplanted on the subcutaneous bed and they are resistant to injury. Secondary contracture/scarring, which occurs due to myofibroblasts on the substrate of the recipient site and can lead to permanent contracture especially on the lower limbs and the joint surfaces, is minimal in fullthickness grafts. Relevant studies of dogs have shown that graft size may increase post healing.5 Hair growth is improved as compared to split-thickness grafts; however, it might not be as thick as the corresponding hair growth of cutaneous flaps, due to damage at the base of the hair follicles located in the subcutaneous tissue that has been removed.15 In general, full-thickness skin grafts in veterinary medicine are meshed.7,13,14
With regard split-thickness grafts, whilst commonly used in human cosmetic surgery, their use in veterinary surgery is limited mostly to extensive skin defects.4,12 They are harvested with a special dermatome which ensures that a certain thickness of skin tissue will be removed.12 They can also be excised by scalpel, though it is usually diffi cult to ensure proper thickness.11 Generally more sensitive to handling than full-thickness grafts, split-thickness grafts require special attention and care during harvesting and placement.6 Depending on the required skin thickness, the preparation process with a dermatome divides the adnexa between the graft and the donor dermis. This results in low tolerance to injury in both the donor and recipient site, greater propensity for contracture, and sparse hair regrowth.6,12 Furthermore, with this particular dermatome technique, the donor site cannot be surgically resurfaced; hence, healing occurs by secondary intention.12 Split-thickness and full-thickness skin grafts have similar viability. However, there are studies in human medicine that support the increased viability of split-thickness skin grafts, which is attributed to the denser capillary network of the exposed dermal vascular plexus compared to the vascu- lar plexus of full-thickness grafts.4,5 Τhe thinner skin of such grafts ensures a shorter distance for plasma diffusion, thereby facilitating improved cellular survival during the stage of plasmatic imbibition.5 Studies have reported that split-thickness skin grafts have the advantage of expansion due to less collagen and elastic fi bres compared to full-thickness skin grafts.5 Grafts of this type are less tolerant and more exposed to injury; hence, their use in lower limb defects in companion animals is limited.3,6 Table 1 outlines the basic characteristics of full and split-thickness skin grafts according to the selection criteria of the proper donor site as well as the required characteristics of the graft.1,4,9,10
> Indications of graft transplantation
Skin grafts are mostly used to resurface extensive skin defects located mainly in the lower extremities of dogs and cats. The most common indications for skin grafts include the following:
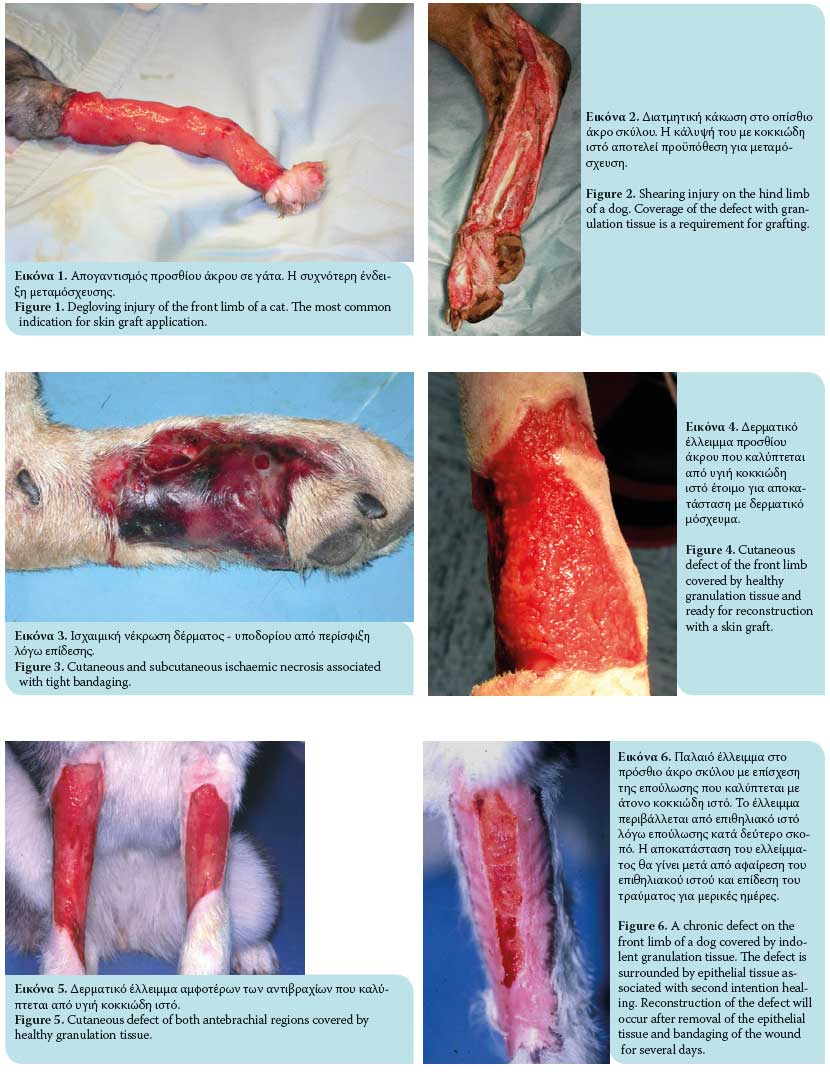
- Degloving injuries due to traffic accidents or bite wounds from other animals characterized by denuded skin on the limbs (Figure 1). Degloving may be mechanical in nature in cases where the epidermis becomes detached from the subcutaneous tissues due to skin entrapment in the wheels of automobiles, or it may be functional where necrosis of extensive areas of skin occurs due to ischaemia of the subcutaneous vascular plexus and bacterial infection. Degloving injuries are initially treated as open wounds; following granulation tissue formation, they are covered by skin grafts.1,6,7,11,13
- Shearing wounds (Figure 2).7,11
- Recent surgical wounds created by the removal of extensive tumours of the skin and subcutaneous tissue.7,14
- Cellulitis or necrotizing fasciitis.7
- Ischaemic bandage injuries (Figure 3).7,11
- Reptile or arthropod bites.7
- Skin burns that occasionally occur in animals and are mainly located on the trunk and less commonly on the limbs.1,3,12
- Defects caused by toxic epidermal necrolysis.
> Graft harvesting and reconstruction technique
Selection of the donor site
In general, the donor site should be capable of supplying adequate skin for the transplantation, and closure should be possible by approximating and suturing the wound edges. In veterinary practice, the most common skin donor site is the area of the lateral thoracic wall.7,13,14 This is due to the fact that there is adequate loose and thin skin tissue in these areas to facilitate closure of the surgical wound formed after the excision of the graft and achieve a satisfactory cosmetic result. If this area is unavailable, alternate areas for graft harvesting include the abdominal and cervical skin, avoiding the thicker skin tissue of the dorsal aspect, because of delayed revascularization.6 However, the abdominal skin contains comparatively less hair follicles and is therefore generally avoided.6 Skin from the inguinal region usually contains hair growth and skin tissue of supreme quality.6 The scrotum has also been used as a full-thickness skin graft for the resurfacing of skin defects in dogs.16,17,18
Preparation of the recipient site
To secure a successful graft take, the recipient site should be free of contamination and exudate and have an adequate vascular supply. Therefore, the presence of healthy granulation tissue is necessary (a wound bed environment free of pathogen contamination and with adequate blood perfusion); it is also vital for graft revascularization (Figures 4, 5, 6). The use of skin grafts is further indicated in the reconstruction of surgical wounds.14 Twenty-four hours prior to grafting, it is recommended that the granulation tissue of the recipient site be activated by mechanical debridement with hot or cold dressings, or with surface debridement with a scalpel intended to remove surface contaminants and pathological granulation tissue. Successful skin grafting requires a wound bed with a rich vascular supply, close contact of the graft interface and wound bed, and adequate preparation of the granulation tissue in the subcutaneous bed.1-3,6 On the day of the procedure, any layer of epithelial tissue is removed from the wound edges with a no. 15 scalpel at the borderline between hairy skin and epithelial layer.2 Graft size is calculated by measuring the dimensions of the recipient site. For this purpose, a gauze dressing is placed on the surface of the surgical site or the surface of the healthy granulation tissue; in this way, a «blood imprint» is created so as to be transported to the recipient site.
Preparation and application of the graft
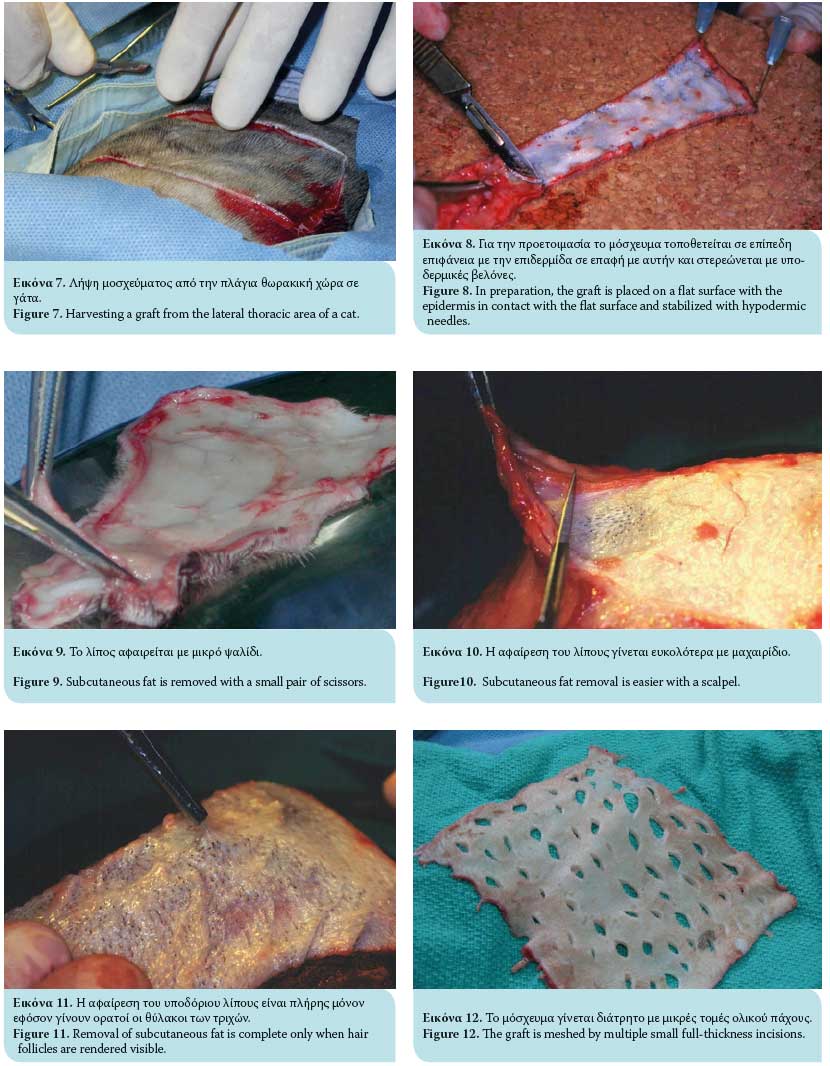
Once the full-thickness graft is harvested, the residual adipose subcutaneous tissue is removed, usually with a small pair of scissors or by excision with a no 10 scalpel (Figures 7, 8, 9, 10). Removal of adipose tissue is considered to be complete once hair follicles are revealed (Figure 11). Subsequently, multiple small incisions are made with a no. 11 scalpel blade, 1-2 cm in length and within 0.5-2 cm of each other, resulting in the creation of a meshed graft (Figure 12). The formation of a meshed graft allows drainage of the exudate, permits the expansion of the graft, and facilitates the application and fixation of the graft to the recipient site.1-3,6,7,10,13-15
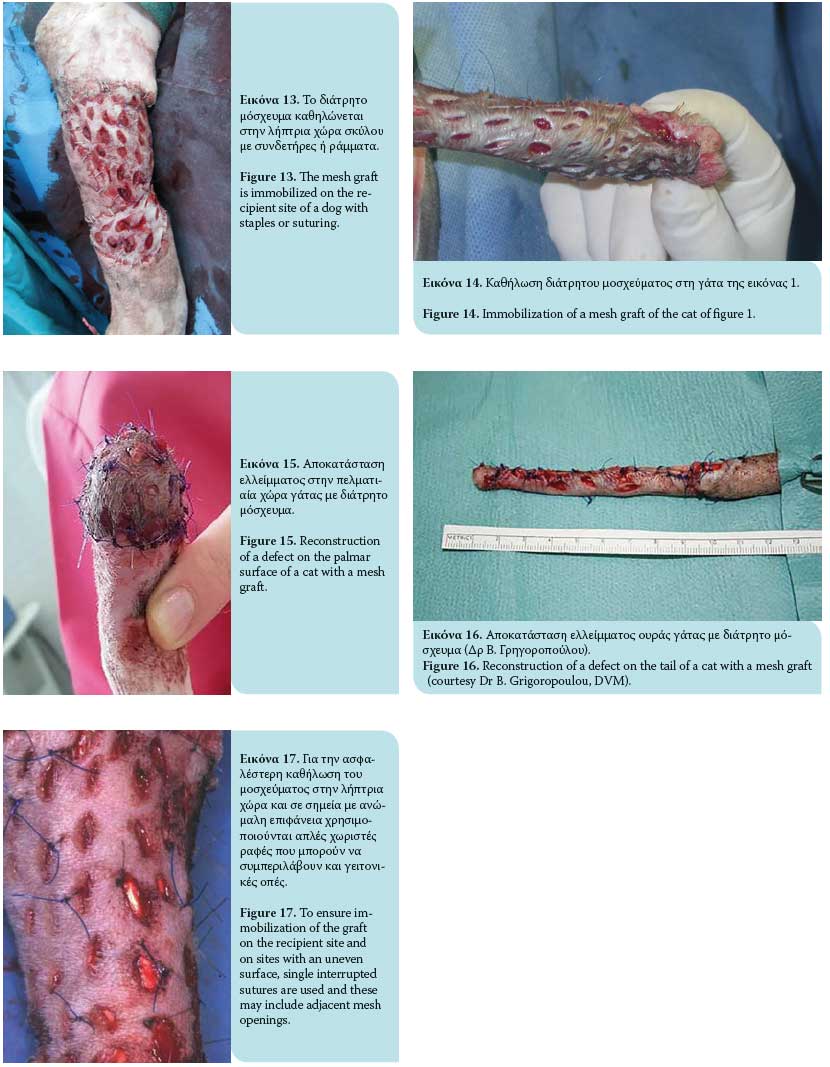
Τhe graft is then ready to be transferred to the recipi- ent area. It is recommended that a direction of hair follicles be maintained parallel to the remaining fur of the target area during grafting. Immobilization of the graft at the recipient site is accomplished by 3/0- 4/0 nylon simple interrupted sutures or surgical staples that approximate the graft edges to the skin of the recipient site. Single interrupted sutures are used as a more effective method of securing the graft to the recipient site and where the surface is uneven (e.g. bone protuberances) and these can include adjacent incision openings (Figures 13, 14, 15, 16, 17). The wound edges of the donor site are closed primarily by apposition of the skin edges with sutures. In a retrospective study of skin grafts in 52 canine and feline cases, the most common recipient sites were the metatarsals, tarsal joints, metacarpals, and carpal joints.7
> Pathophysiology of the graft take
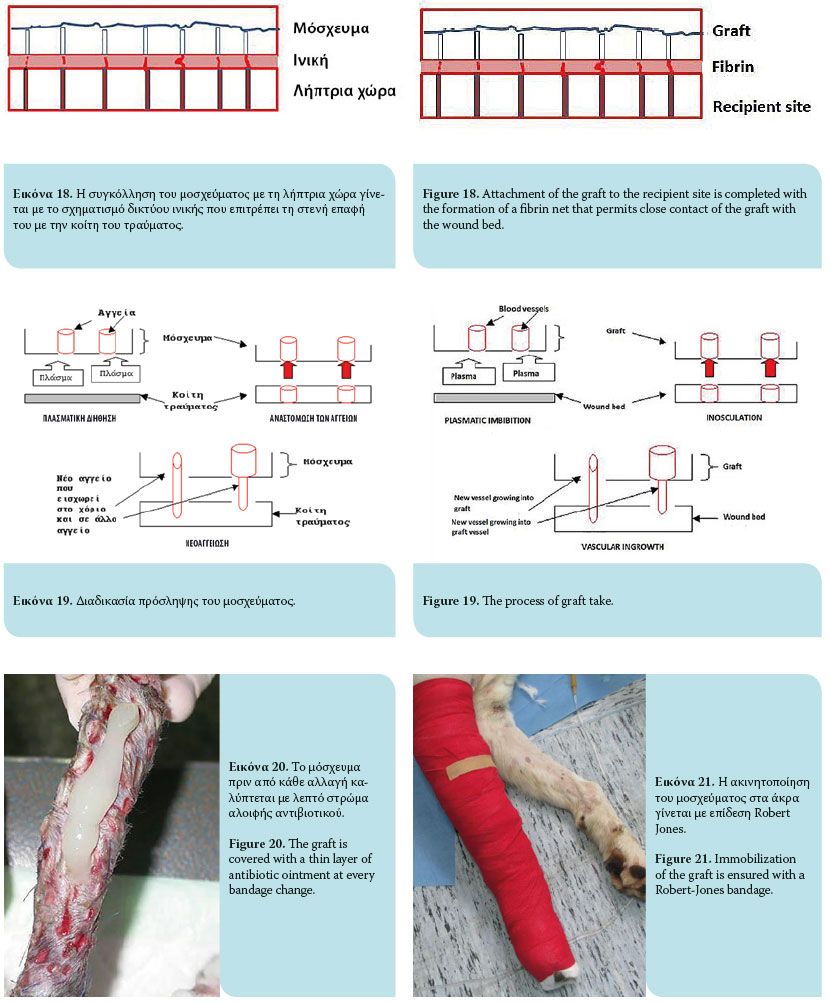
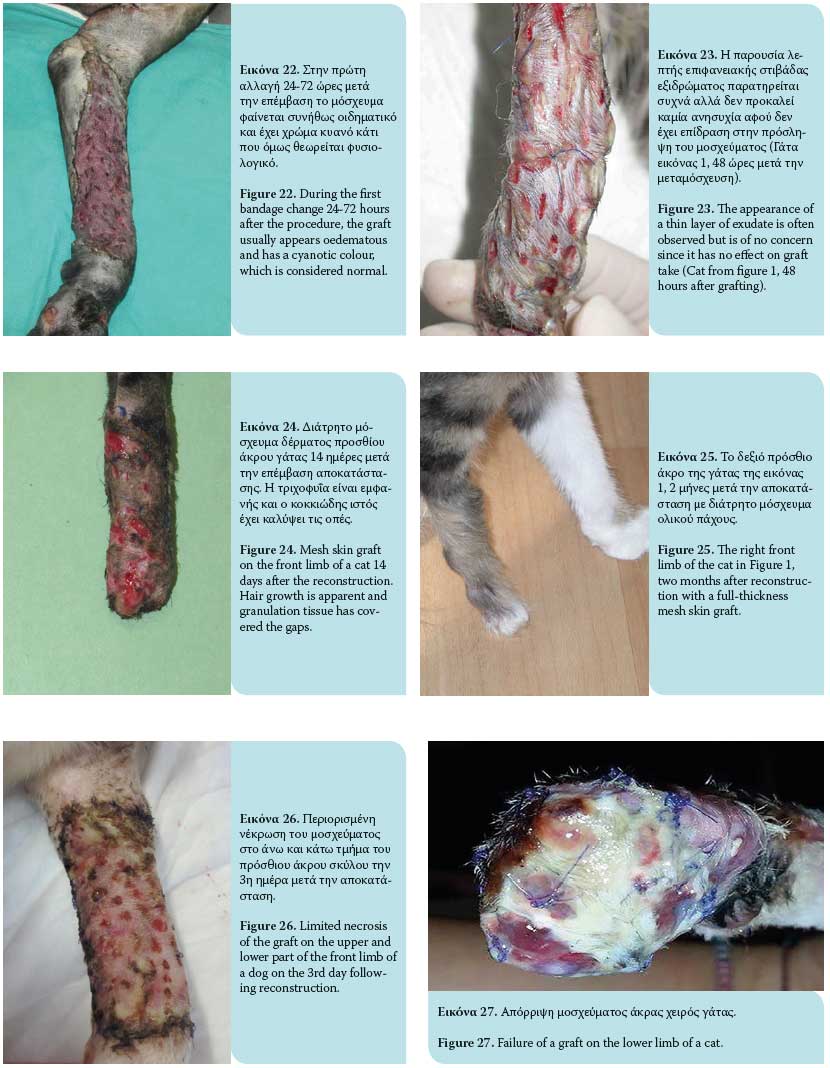
The graft take process initiates as soon as it is placed on the recipient site and lasts for 15 days. This process includes graft adherence, plasmatic imbibition from the recipient bed to the dilated blood vessels of the graft, inosculation (vascular anastomosis of graft vessels with wound bed vessels) and vascular ingrowth (revascularization of the graft from the recipient bed) [Figures 18, 19].1,3,4,6,9
Graft adherence to the recipient bed is accomplished by formation of a fibrin network, which allows close contact of the graft with the recipient area. Initially, the fibrin network comprises the stromal interface between the collagen and elastin of the graft and the wound bed. Within 8 hours, contact of polymerized fibrin with the graft and the recipient site intensifies and is strengthened. Within 72 hours of transplantation, the fi brin network is transformed into fibrous tissue, after which it is infused with fi broblasts, white blood cells and phagocytes, resulting on the 10th day in complete adherence.1,3,4,6,9
Plasmatic imbibition occurs immediately after graft application, when serum, red blood cells and neutrophils leaking from the blood vessels of the recipient bed aggregate between the graft and the recipient site. The graft blood vessels dilate and passively absorb plasma into the graft through capillary action. A result of this phenomenon is the quick nourishing of the graft until its revascularization. Absorption of haemoglobin products gives a blue colour to the graft during the fi rst 48 hours. The aggregation of fluid that diff uses into the extracellular matrix causes oedema, which will recede to a signifi cant degree in time and after reconstruction of veins and lymphatic drainage.1,3,4,6,9
The inosculation process is the anastomosis of the graft blood vessels with the vessels of the same diameter of the recipient bed, generally observed within 48-72 hours after grafting. The fibrin network functions as a scaff old through which vascular branches of the wound bed vascular plexus advance to reach the vessels of the graft base, with which they finally unite. Minimal blood flow initiates in graft vessels by the 3rd to 4th day post grafting and continues to develop until normal blood fl ow is restored by the 5th to 6th day after the procedure.4 However, this blood flow may easily be arrested. Given that the anastomosing process occurs randomly, veins of the graft can be united with arteries of the wound bed and vice versa. This results in reformation of the blood vessels.1,3,4,6,9
Vascular ingrowth involves revascularization of the graft generated by the invasion of graft vessels by wound bed blood vessels, thereby creating new endothelial pathways and ingrowth of the vascular plexus of the recipient bed in pre-existing endothelial pathways of the graft. Shortly after graft application on the recipient site, a highly vascular granulation tissue develops. This results in the formation of new capillaries through which blood flows; this process commences 18 to 24 hours after the procedure. New blood vessels grow from the endothelial tissue. Such growths may develop from arterioles and venules. Once the continuity between the old and new blood vessels is restored, the newly-formed blood vessel is filled with red blood cells. Newly-formed blood vessels undergo abnormal distension and undertake a temporary tortuous appearance. Maturation of new, undiff erentiated capillaries begins within 48 hours of their appearance. Blood vessels subsequently straighten and form new arterioles by receiving increased amounts of blood. This differentiation and maturation process continues until a network of arterioles, veins and capillaries is formed. The rate of inner development of new capillaries has been calculated to be about 0.5 mm/day. 1,3,4,6,9
Revascularization of the graft may also occur by ingrowth of new blood vessels within the pre-existing graft vessels following the path of least resistance leading to the rapid growth of blood vessels. The ingrowth process may proceed if there is contact of endothelial cells with viable endothelial tissue in old graft vessels. Blood vessels of the graft that are not involved in inosculation or ingrowth are degenerate and dissolve.1,3,4,6,9
Full-thickness grafts acquire re-innervation randomly. Consequently, in most grafts, the periphery is normally innervated whereas central areas reduce or become void of sensation. The re-innervation process begins from the periphery of the graft and moves toward the interior by invading neural fibres mostly by following the vacated Schwann sheaths. Accessibility of Schwann sheaths to the invading neural fibres determines the extent of the re-innervation process. Invading neural fi bres, which are not connected to Schwann sheaths may transverse only a short distance within the graft. Pain is the fi rst sign that sensation is returning, followed by response to touch and later the perception of heat. At the fi nal stage, hypersensitivity transpires; however, sensation eventually returns to normal. In studies performed in pigs, the re-innervation process begins at 3-4 weeks and by 7-8 weeks it reaches 50%.1,3,4,6,9
> Post surgical care
During the process of graft take, the graft should be protected from infection and the area should be immobilized. This is accomplished by adequate surgical sterilization, bandaging, frequent dressing changes and antibiotic administration. The graft is covered by a thin layer of an antibiotic ointment at every bandage change (Figure 20). A non-adherent patch is applied, made either of paraffin (Jelonet, Smith & Nephew), acrylic polyester fi bre (Melolin, Smith & Nephew), polyurethane (Hydrofi lm, Hartmann, Alevyn, Smith & Nephew), polyethylene (Cosmopor, Hartmann) or propylene with cellulose particles (Zetuvit E, Hartmann), and the area is wrapped with a roll gauze followed by an adhesive elastic bandage to secure adequate immobilization.7,13,14 Immobilization is usually achieved with a Robert Jones bandage or rarely, in the case the graft over a joint, with external fi xation (Figure 21). The fi rst bandage is changed within 48 - 72 hours of grafting. After the first bandage change, the graft usually appears to be oedematous with a cyanotic colour, but this is considered normal (Figure 22). In the following days, oedema resolves and the graft appears pink due to restoration of local blood perfusion. The presence of a thin surface layer of exudate is commonly observed but without cause for concern since it has no eff ect on graft take (Figure 23). Hair growth occurs in the 2nd to 3rd week after grafting (Figures 24, 25). Dressing are initially changed daily or every other day, and later more infrequently, depending on the amount of fluid produced. During bandage change, the graft is lightly cleansed with sterilized cotton buds and normal saline.2,7,10,13,14
> Complications
The most severe complication of grafting is failure. Graft detachment from the recipient bed resulting in breakdown of the fibrin bands connecting the graft to the wound bed leading to arrest of revascularization and failure of graft nourishment (Figures 26, 27). The appearance of a white or black colour on the graft is a sign of failure. Predisposing factors for failure include the formation of a seroma or haematoma that could cause detachment of the graft from the wound interface, dehiscence, and infection of the graft. In contrast, partial or full epidermal necrosis should not necessarily be a cause for concern since in most cases the dermis becomes attached to the recipient bed and is still viable.1-4,6
Relocation (slippage) of a cutaneous graft can be prevented with correctly placed sutures around the periphery of the graft, as well as extra suturing at strategic locations, as well as immobilizing the graft with bandages. Bacteria can cause destruction of the newly formed fi brous tissue or produce enough exudate to lift up the graft from the wound bed. Infection is usually caused by Pseudomonas spp or Klebsiella spp. It is therefore mandatory that aseptic technique guidelines be followed throughout the transplantation process, both perioperatively and postoperatively. In addition, irrigation of the skin graft with antibiotic solutions can reduce the possibility of infection. Local application of antibiotics in a spray form that are eff ective against microorganisms of the Pseudomonas species and β-lactamase-producing bacteria, has off ered satisfactory results. If necessary, a systemic course of antibiotics is implemented, with care in choosing the appropriate antibiotic, so that the re-epithelisation process is not arrested.1-4,6 In case of failure, removal of the necrotic tissue is mandatory.
In the largest retrospective study to date regarding skin grafting with full-thickness skin mesh grafts in 32 dogs and 20 cats, 77% of the grafts in cats survived in contrast to 38% of the grafts in dogs and the difference was statistically signifi cant.7 An experimental study on 24 male intact dogs showed that 50% of scrotum grafts still survived.17 Furthermore, scrotum graft take on the recipient bed had a much longer duration than that of other cutaneous grafts, whereas hair growth was insuffi cient and there was a clear difference in hair colour compared to the recipient site.17
> References
1. Swaim SF: Skin grafts. Vet Clin North Am. Small Anim Pract 1990, 20: 147-175.
2. Swaim SF. Skin grafts. In: Management of Small Animal Distal Limb Injuries. Swaim SF, Welch J, Gillete RL (eds).Teton New Media: Jackson, 2015, pp. 154-161.
3. Bohling MW, Swaim SF: Skin grafts. In: Veterinary Surgery: Small Animal. Tobias KM, Johnston SA (eds). Elsevier: St Louis, 2012, pp. 1271- 1290.
4. McGregor AD, McGregor IA: Free skin grafts. In: Fundamental Techniques of Plastic Surgery and their Surgical Applications. McGregor AD, McGregor IA (eds). 10th edn. Churchill Livingstone: Edinburgh, 2000, pp. 35-59.
5. Bauer MS, Pope ER: The eff ects of skin graft thickness on graft viability and change in original graft area in dogs. Vet Surg 1986, 15: 321-324.
6. White RAS: Free skin grafting. In: Manual of Canine and Feline Wound Management and Reconstruction. Williams J, Moores A (eds). 2nd edn. BSAVA: Gloucester, 2009 pp. 144-158.
7. Riggs J, Frazer Jennings JL, Friend EJ, Halfacree Z, Nelissen P, Holmes MA, Demetriou JL. Outcome of full – thickness skin grafts used to close skin defects involving the distal aspects of the limbs in cats and dogs: 52 cases (2005-2012). J Am Vet Med Assoc 2015, 247: 1042-1047.
8. Fowler JD, Miller CW, Bowen V, Johnston GH. Transfer of free vascular cutaneous fl aps by microvascular anastomosis results in six dogs. Vet Surg 1987, 16: 446-450.
9. Pope ER: Skin grafting in small animal surgery. Part I. The normal healing process. Compend Contin Educ Pract Vet 1988, 10: 915-923.
10. Pope ER. Skin grafting in small animal surgery. Part II. Full-thickness skin-grafting techniques. Compend Contin Educ Pract Vet 1988, 10: 1068- 1077.
11. Shahar R, Shamir MH, Brehm DM, Johnston DE. Free skin grafting for treatment of distal limb skin defects in cats. J Small Anim Pract 1999, 40: 378-382.
12. Aragon CL, Harvey SE, Allen SW, McCrackin MA. Partial-thickness skin grafting for large thermal skin wounds in dogs. Compend Contin Educ Vet, 2004, 26: 200-215.
13. Siegfried R, Schmοkel H, Rytz U, Spreng D, Schwalder P. Treatments of large distal extremity skin wounds with autogenous full-thickness mesh skin grafts in fi ve cats. Schweiz Arch Tierheilk 2004, 146: 277-283.
14. Tong T, Simpson DJ. Free skin grafts for immediate wound coverage following tumor resection from the canine distal limb. J Small Anim Pract, 2012, 53: 520-525.
15. Pope ER, Swaim SF. Wound drainage from under full-thickness skin grafts in dogs. Part II. Eff ect on cosmetic appearance. Vet Surg 1986, 15: 72-78.
16. Harris JE, Dhupa S. Treatment of degloving injuries with autogenous full thickness mesh scrotal grafts. Vet Comp Orthop Traumatol 2008, 21: 378-321.
17. Grigoropoulou VA, Prassinos NN, Papazoglou LG, Galatos AD, Psalla DA. The use of canine scrotum as a mesh graft to cover skin defects. In: Proceedings Third World Veterinary Orthopaedic Congress. Bologna, Italy, 2010, pp. 572-573.
18. Wells S, Gottfried SD. Utilization of the scrotum as a full thickness skin graft in a dog. Can Vet J 2010, 51: 1269-1273.



