> Abstract
Εrythrogram is part of the complete blood count and includes the number of erythrocytes, hemoglobin concentration, hematocrit, erythrocyte indices (mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration), red cell distribution width, the number and percentage of reticulocytes and erythrocyte morphology. Appropriate blood sampling and handling are essential for the validity of the erythrogram interpretation. Erythrogram major abnormalities are anemia, erythrocytosis/polycythemia, erythrocyte morphologic abnormalities and erythrocyte inclusions. Anemia can be either regenerative or nonregenerative. Regenerative anemia is divided into blood loss anemia and hemolytic anemia. Common causes of blood loss anemia are traumatic injuries and hemostatic disorders, while hemolytic anemia can be immune-mediated, microangiopathic, associated with Heinzbody formation or due to genetic defects of red blood cells. Nonregenerative anemia includes anemia of chronic disease, anemia of chronic renal failure, aplastic anemia and nutritional anemia. Erythrocytosis/polycythemia can be relative (hemoconcentration) or absolute, which is further divided into primary (polycythemia vera) and secondary. The appearance of abnormal erythrocytes can be either an artifact or associated with certain disorders. The erythrocyte inclusions are either of non-infectious origin, such as Howell-Jolly and Heinz bodies or related to infections, such as Babesia spp. and hemotropic Mycoplasma spp., the observation of which into the erythrocytes sets the defi nitive diagnosis of the relevant diseases.
> Introduction
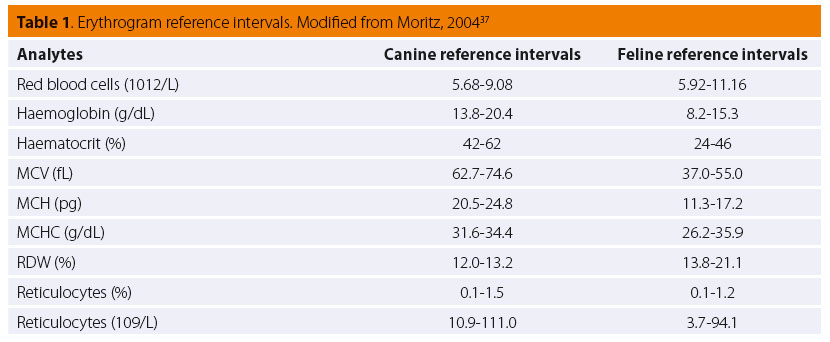
Εrythrogram is part of the complete blood count (CBC) and includes red blood cells (RBC) count, hemoglobin concentration, hematocrit, red blood cell indices [mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC)], red cell distribution width (RDW), reticulocytes percentage and count and the evaluation of erythrocyte morphology on stained blood smears (Table 1). Appropriate blood collection, sample handling and storage are essential for the reliability of the results. Overnight fasting should precede blood collection in order to prevent postprandial lipemia, which can interfere with the measurement of plasma hemoglobin concentration.1 The blood should be collected into EDTA tubes and fi lled up to the defi ned limit in order to avoid blood clotting (overfi lled tubes) or decrease of the hematocrit (underfi lled tubes).2 Avoidance of iatrogenic blood hemolysis, due to poor blood collection technique or sample handling, is critical because it can interfere with various erythrocyte parameters. The CBC and preparation of blood smears should be completed as soon as possible, preferably within 2-3 hours after blood collection. However, blood in EDTA tubes can be safely stored in the refrigerator for 24 hours.1,3 Refrigerated blood samples should be kept at room temperature and be gently inverted before analysis. It should be noted that inappropriate drying, fi xation and staining can lead to low-quality stained blood smears. The most commonly used stains are the Romanowsky-type (for routine morphologic evaluation of blood cells), new methylene blue (for the determination of reticulocytes number and presence of Heinz bodies) and Prussian blue (for the verifi cation of iron-containing inclusions in blood cells).
> Anemia
Anemia is defi ned as decreased hemoglobin concentration, which is typically associated with a decreased number of circulating RBCs and low hema- tocrit.1 The above mentioned pathologic state, also named absolute anemia, should be diff erentiated from relative anemia, which is the result of hemodilution.4 Anemia is classifi ed by bone marrow responsiveness (regenerative, nonregenerative), erythro- cyte indices (microcytic, normocytic, macrocytic and hypochromic, normochromic) and pathophysiology (anemia due to a decreased red blood cell life span and anemia caused by decreased erythrocyte production).4,5 However, the ultimate goal is the classifi cation of anemia according to the etiology.
> Regenerative anemia
Regenerative anemia is characterized by reticulocytosis, which is the best marker for accelerated erythropoiesis, and hence by anisocytosis (increased RDW), macrocytosis (increased MCV), hypochromasia (decreased MCHC) and polychromasia (highly indicative of regeneration).6,7 Regenerative anemia is divided into two major categories: blood loss anemia (BLA) and hemolytic anemia.
BLA is characterized by moderate regeneration, although it may be nonregenerative in early stages and poorly regenerative or even nonregenerative in late stages. In the first 2-4 days, blood loss anemia is nonregenerative, normocytic and normochromic, because reticulocytosis is observed 2-4 days after the onset of the hemorrhage.6,8 In the next stage, anemia becomes regenerative, macrocytic and hypochromic and remains as such, unless iron defi ciency anemia is developed secondary to chronic hemorrhage. The most common causes of BLA are traumatic injuries (including those surgicallyinduced) and hemostatic disorders, which occur as a result of severe thrombocytopenia, anticoagulant intoxication, functional defects of platelets (uremia, severe liver disease, specifi c drug administration, etc.) or inherited disorders (such as hemophilia A, B and von Willebrand’s disease).9 Neoplasms, especially hemangiomas and hemangiosarcomas in the spleen, liver and lungs, as well as those of the gastrointestinal or urinary tract are strongly associated with chronic blood loss.9 Parasitic infections with ectoparasites (e.g. fl eas, lice and ticks) or endoparasites (e.g. Ancylostoma spp, Uncinaria spp and coccidia), especially in young and small dog breeds, gastrointestinal ulcers, foreign bodies (particularly in the gastrointestinal tract), and urinary calculi are also reported as causes of BLA.9
Hemolytic anemia is a condition in which the rate of erythrocyte destruction is higher than the erythrocyte production in the bone marrow. It is the regenerative anemia type with the greatest response.6,8 However, in some cases, such as bone marrow damage or production of antibodies against RBC precursors in bone marrow, the regeneration is poor to none.9 Hemolysis can be intravascular (within the blood vessels or heart) or extravascular (destruction of erythrocytes in the macrophages of the spleen, liver and bone marrow). Hemolytic anemia could be classifi ed into four main categories. Hemolytic anemia due to RBCs immune-mediated damage, mechanical damage (microangiopathic hemolytic anemia), oxidative damage (Heinz body anemia) and because of a heterogeneous group of inherited defects.
Immune-mediated hemolytic anemia (IMHA) can be primary or secondary, due to immune disorders, infectious and neoplastic diseases or drug administration. Primary (autoimmune hemolytic anemia) is much more common in dogs than in cats.4 Infectious agents can cause hemolytic anemia directly by attacking erythrocytes or indirectly by the eff ect that their products have on erythrocytes. IMHA may be seen in cases of leishmaniosis, ehrlichiosis, dirofi lariasis, hemotropic mycoplasmosis and babesiosis.6,10 Immune-mediated hemolysis has been observed in lymphoproliferative diseases, such as lymphomas and leukemias.6 Drugs that are commonly used in clinical practice and potentially cause IMHA are antibiotics (penicillins, cephalosporins, trimethoprim-sulfonamide), non-steroidal antiinfl ammatory drugs (paracetamol), antiarrythmics, etc.4,10 Immune-mediated hemolysis may also be seen in transfusion reactions, vaccine administration and neonatal isoerythrolysis.11
The mechanical injury of RBCs (microangiopathic hemolytic anemia) is the result of certain pathologic conditions and diseases, such as vasculitis, disseminated intravascular coagulation (DIC) (anemia due to DIC may also be hemorrhagic due to multiple hemorrhages), hemangiosarcoma, dirofi lariasis and cardiac, hepatic or splenic disease.4,6,10
Heinz body anemia is a result of a variety of factors, which may be easily identifi ed from the history of the animal patient. Specifi c drug administration (e.g. paracetamol, benzocaine), as well as the consumption of onion, garlic and their products, are associated with this type of hemolytic anemia.4,12,13 Heinz body anemia can emerge in the progress of some endocrine diseases, such as diabetes mellitus and hyperthyroidism, as well as in neoplastic diseases such as lymphomas.10,12,14 Furthermore, chemicals (e.g. heavy metals, propylene glycol, phenolics) are also associated with the onset of Heinz body anemia, while hemolytic anemia which accompanies hypophosphatemia may occasionally be related to Heinz body formation.4,6,12
Hemolytic anemia due to inherited genetic defects of RBCs are rarely encountered in clinical practice and include erythrocyte membrane defects (e.g. hereditary elliptocytosis, hereditary stomatocytosis), erythrocyte enzyme abnormalities (e.g. phosphofructokinase defi ciency, pyruvate kinase defi ciency, feline porhyria, methemoglobin reductase defi ciency) and other pathologic conditions, such as familiar non-spherocytic hemolytic anemia (as reported in Beagle and Poodle), in which there is no evidence of disorders in morphology or in the enzymes of erythrocytes.4,15-18
> Non-regenerative anemia
Non-regenerative anemia is the most common type of anemia in clinical practice.19 This anemia may arise either because of abnormalities of the stem cells, their modulators and the bone marrow environment or because of disorders in the later-stage regulators of erythroid proliferation, including erythropoietin, cytokines and iron.19 As the average life span of erythrocytes is 100-120 days in dogs and 70- 78 days in cats, the development of anemia, which is only caused by decreased erythropoiesis, might take several weeks or even months.19 Reticulocytes are either not seen in great numbers or not seen at all in circulation; hence, non-regenerative anemia is characterized as normocytic and normochromic.9,20 Exceptions include nutritional anemias, anemias associated with myeloproliferative disorders, and certain cases with anemia of chronic disease.9 Non- regenerative anemia can be classifi ed into five major categories: i) anemia of chronic disease (ACD), ii) erythropoietin-related anemia (ERA), iii) anemia due to bone marrow disorders, iv) nutritional defi ciency anemia and v) anemia due to acute and peracute blood loss or hemolysis (fi rst 48 to 96 hours).
ACD (also called anemia of infl ammation) is the most frequently encountered anemia in small animals.19 The production of cytokines during infl ammation results in the decrease of iron availability, erythropoietic response and RBCs life span.19 Typically, laboratory evaluation reveals mild to moderate normocytic and normochromic anemia.18 Moreover, anemia is accompanied by decreased levels of serum iron and serum transferrin and normal or increased serum ferritin concentration.19,20 ACD can be a result of both infectious (bacterial, viral, protozoal, fungal) and non-infectious (immunological, neoplastic) diseases.4 Regarding anemia due to neoplasia, it should be mentioned that this can also be a consequence of hemorrhage, hemolysis, myelophthisis, myelofi brosis and myelodysplasia.19-21
ERA has as its main pathogenic mechanism the suppression of erythropoietin and can be caused either by chronic renal disease or by specific endocrine disorders. Anemia of chronic renal disease (or chronic renal failure), which refers to end-stage renal disease or renal failure, is mainly a result of erythropoietin defi ciency and uremia.19,22 This type of anemia is mild to moderate, normocytic, normochromic, with normal iron distribution, unless there is evidence of infl ammation.19 ERA can be developed secondary to endocrine disorders, such as hypothyroidism, hypoadrenocorticism and pituitary dwarfi sm.19 The anemia due to hypothyroidism or hypoadrenocorticism is usually mild, normocytic and normochromic.23
Non-regenerative anemia can be the result of the following bone marrow disorders: bone marrow or erythroid aplasia/hypoplasia [known as aplastic anemia and pure red cell aplasia (PRCA), respectively], myelophthisis, myelodysplasia and myelofibrosis. All these disorders, apart from pure red cell aplasia, affect two or even all three cell lineages in the bone marrow; thus, leukopenia and/or thrombocytopenia are also present along with anemia. Pure red cell aplasia (PRCA) leads to a severe, normocytic, normochromic anemia, with absolute reticulocytopenia and the virtual absence of bone marrow erythroid precursor cells.24 PRCA can be immune-mediated (it is considered a type of nonregenerative ΙΜΗΑ), related to viral infections [canine parvoenteritis, feline leukemia virus (FeLV) infection] or rarely congenital.24-26 Aplastic anemia (also called aplastic pancytopenia) is characterized by bicytopenia or pancytopenia and replacement of normal bone marrow elements by adipose tissue.27 Infectious diseases, such as ehrlichiosis, parvoenteritis, leishmaniosis, histoplasmosis in dog and FeLV, feline immunodefi ciency virus (FIV) infections and panleukopenia in cat, frequently cause aplasia of bone marrow.27 Hyperestrogenism, either of endogenous origin (e.g. Sertoli cell neoplasia) or of exogenous-iatrogenic origin caused by an overdose of estrogens, is a causative factor for both dogs and cats, although dogs are much more susceptible to high levels of estrogens.19,27 Transient aplastic anemia is reported in radiation syndrome and after the administration of specifi c drugs, such as chemotherapeutics (azathioprine, cyclophosphamide, etc.), phenylbutazone, sulfadiazine, griseofulvin, chloramphenicol and fenbendazole.18,19,27,28 If an etiologic diagnosis cannot be established, aplastic anemia is defi ned as idiopathic.29 Myelophthisic anemia develops secondary to a space-occupying lesion in the bone marrow where non-marrow elements, such as neoplastic, infl ammatory and stromal cells, crowd out the normal hematopoietic precursors of bone marrow.19 The laboratory evaluation shows a mild to severe, normocytic, normochromic anemia, reticulocytopenia and leukoerythroblastic reaction.19 Diseases associated with myelophthistic anemia are hematopoietic or metastatic neoplasms and granulomatous infl ammatory diseases.18,20 Myelodysplastic syndromes (MDS) are a heterogeneous group of hematopoietic stem cell disorders characterized by cytologic dysplasia in the blood and bone marrow and by various combinations of anemia, neutropenia and thrombocytopenia.19 Myelodysplasia is seen in both dogs and cats, but is more common in cats with FeLV infection.20 In dogs, myelodysplasia can be genetically-based or secondary to drug administration.19 All MDS are associated with non-regenerative, normocytic and normochromic anemia.30 Myelofi brosis is the replacement of normal elements of bone marrow from fi brous tissue produced by fi broblasts.20 Myelofi brosis may lead to another similar condition, called osteosclerosis or myelosclerosis, in which instead of fi brous tissue, osseous tissue is produced by osteoblasts.20,31 Both syndromes are rare and have been observed in FeLV infection in cats, in dogs with congenital pyruvate kinase defi ciency, in carcinomas and in cases without any underlying condition (idiopathic).20 Most patients with these syndromes are severely anemic and present leukoerythroblastic reaction.19
Nutritional defi ciency anemia is caused by a prolonged lack of minerals, vitamins and proteins essential, for the production of erythrocytes.18 By far the most frequent anemia of this group is iron deficiency anemia (IDA). Iron is required for the synthesis of hemoglobin and therefore iron defi ciency can cause anemia, which is classifi ed as non-regenerative, even though mild to moderate regeneration is often observed.32 Although dietary defi ciency is the major cause of IDA in humans, in small animals it is rare and almost always concerns puppies and kittens that are exclusively fed on milk.32 IDA is caused mainly by external hemorrhage and also by insufficient intestinal absorption of iron.32 Regarding RBC indices, acute IDA is normocytic and normochromic and only after the lapse of several weeks or months does it turn into microcytic and hypochromic.9,32 At this point, it should be noted that microcytosis is normally seen in some dog breeds, such as Akita and Sharpei.20 Apart from iron, copper defi ciency can also lead to anemia, which, in contrast to IDA, is normocytic and normochromic.18,32 Vitamin deficiencies, usually observed in animals suff ering from gastrointestinal or pancreatic diseases, constitute rare causes of anemia in dogs and cats. Vitamin B12 and folic acid defi ciency cause macrocytic or normocytic, normochromic anemia, which can be called megaloblastic.9,18 Niacin defi ciency also causes macrocytic and normochromic anemia5. Pyridoxine (vitamin B6) and ribofl avin (vitamin B2) defi ciencies are very rare and constitute etiologic factors of microcytic and hypochromic anemia.9
> Erythrocytosis and polycythemia
Erythrocytosis is defi ned as an increase in hemoglobin concentration, RBC count and hematocrit above the established reference intervals.33 The terms erythrocytosis and polycythemia are often used to describe the same pathologic condition, although erythrocytosis implies an increase in the number of erythrocytes and polycythemia an increase in the number of all three cell lines.34 Erythrocytosis is much more frequent in veterinary medicine than polycythemia.34 It is important to know that certain breeds of dogs, such as Greyhounds, Afghan hounds, Salukis and Whippets, have higher hematocrit values (usually low 60s).4 Based on the pathogenesis, erythrocytosis is classifi ed into relative or absolute and furthermore, absolute erythrocytosis is divided into primary (polycythemia vera) or secondary.33
In relative erythrocytosis, the RBC mass is within the normal reference range, but hematocrit is increased because of the decreased plasma volume (hemoconcentration).33 Relative erythrocytosis is mild to moderate and is the most common type of erythrocytosis in dogs and cats.33 Dehydration is the major causative factor of this pathologic state.34 Splenic contraction due to excitement, anxiety or exercise can cause physiologic erythrocytosis, which is mild to moderate, transient, concerns mainly dogs and may mask concurrent anemia.4 In cases where splenic contraction is suspected, a new blood sample should be collected with the minimum of stress.34
Absolute erythrocytosis is defined as an increase in total RBC mass.33 As mentioned above, it is divided into primary (polycythemia vera) or secondary. Polycythemia vera is a chronic myeloproliferative disorder characterized by the autonomous clonal proliferation of hematopoietic progenitor cells.33 Secondary erythrocytosis results from increased production of erythropoietin, which is typically seen in animals with congenital heart abnormalities (e.g. tetralogy of Fallot, patent ductus arteriosus), chronic severe pulmonary disease, in animals living at high altitudes or those that are physically trained (working dogs).4,33,34 Furthermore, renal infl ammatory, neoplastic or other diseases (e.g. amyloidosis) can cause inappropriate secondary erythrocytosis directly, by secreting erythropoietin, or indirectly, through local hypoxia.33,34 Finally, endocrinopathies, such as hyperadrenocorticism and hyperthyroidism are associated with mild erythrocytosis, due to the hormonal stimulation of erythropoiesis.33
> Erythrocyte morphology
In a blood smear stained with a Romanowsky- type stain, four features of RBCs are evaluated: the color, size, shape and, if present, the inclusions. The color (normochromia, hypochromia) and the size (microcytosis, normocytosis, macrocytosis) can be evaluated both quantitatively (RBC indices) and qualitatively (blood smear). Considering the above, only the shape and the inclusions of RBCs will be discussed in this section, as information regarding the connection between the color and size of RBCs with specifi c hematologic disorders has been previously provided, based on RBC indices.
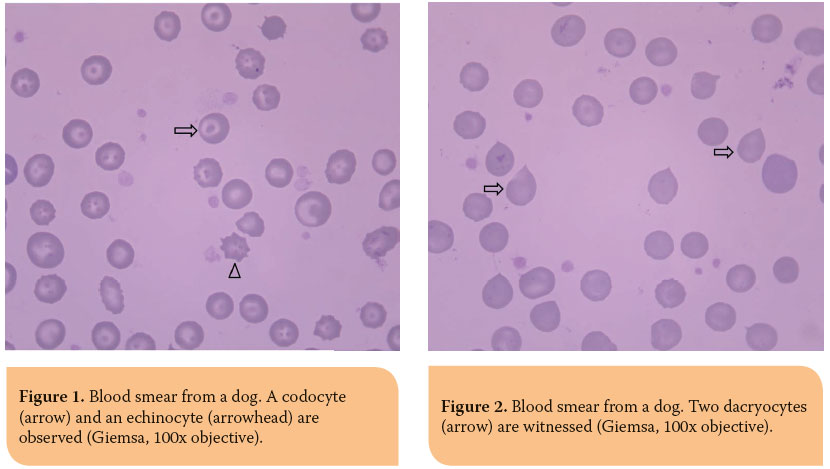
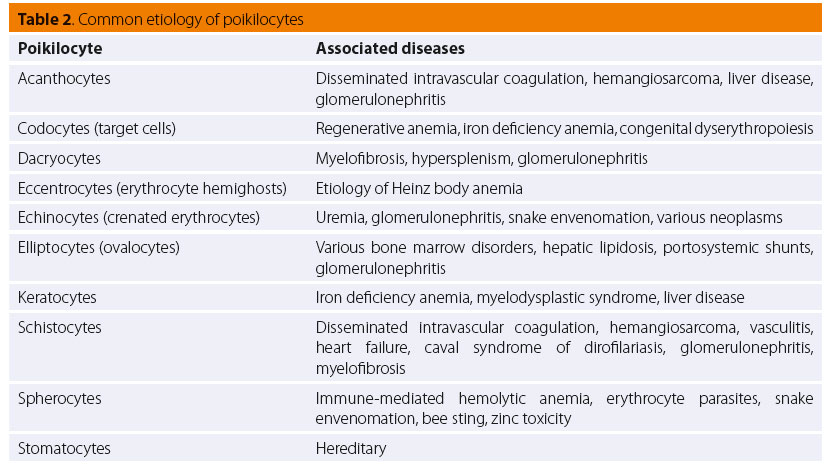
Pathophysiologically, poikilocyte formation is linked to a variety of changes primarily or secondarily aff ecting the erythrocytes. Modifi cations in RBC membrane lipids or proteins are related to the formation of acanthocytes, codocytes (Figure 1), elliptocytes (also called ovalocytes) and echinocytes (although, the latter have a very complex pathogenesis) (Figure 1).35,36 On the other hand, the presence of schistocytes, keratocytes and dacryocytes (Figure 2) in peripheral blood is owed to mechanical damage of RBCs.35,36 Furthermore, erythrocytes oxidative damage and their fragmentation due to phagocytosis leads to the formation of eccentrocytes and spherocytes (Figure 3), respectively, whereas stomatocytes have a hereditary etiopathogenesis.35,36 The presence of echinocytes, dacryocytes and stomatocytes on the blood smear is commonly considered an artifact of inappropriate sample storage or preparation.35,36 The identifi cation of spherocytes is diffi cult in cat, because the RBC central pallor is not prominent, while eccentrocytes are more easily recognized on new methylene blue (NMB) stained preparations.35,36 Finally, it should be mentioned that the concurrent presence of schistocytes, keratocytes and acanthocytes is indicative of lymphoma, hemangiosarcoma, DIC, hepatic cirrhosis, glomerulonephritis or pancreatitis.35,36 Additionally, in the case of snake bites, either echinocytes or spherocytes are produced, depending on the time course and dose of venom.35,36 Further information concerning the etiology of the formation of the various poikilocytes is provided in Table 2.4,35,36
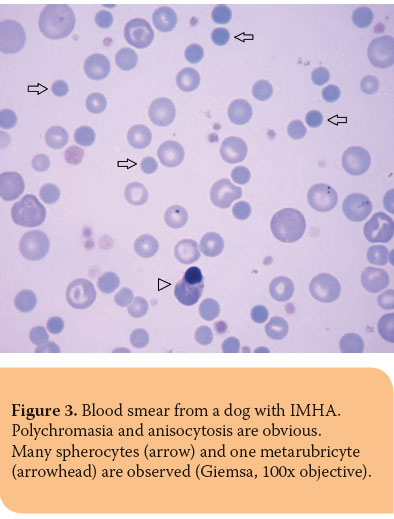 Nucleated RBCs (Figure 3), concurrently with reticulocytes, are seen most frequently in patients with regenerative anemia (Table 3). Spleen or bone marrow diseases, such as hematopoietic neoplasms, myelofi brosis and MDS, are associated with an increased number of circulating nucleated RBCs including less mature erythrocyte precursors, such as rubriblasts and prorubricytes, while lead toxicity is related to metarubricytosis, which is usually not accompanied by anemia.35,36
Nucleated RBCs (Figure 3), concurrently with reticulocytes, are seen most frequently in patients with regenerative anemia (Table 3). Spleen or bone marrow diseases, such as hematopoietic neoplasms, myelofi brosis and MDS, are associated with an increased number of circulating nucleated RBCs including less mature erythrocyte precursors, such as rubriblasts and prorubricytes, while lead toxicity is related to metarubricytosis, which is usually not accompanied by anemia.35,36
Howell-Jolly bodies are nuclear remnants which are observed in the erythrocyte cytoplasm and appear as round, clearly basophilic inclusions which vary in size. They are normally removed by the spleen, therefore low numbers may be seen in healthy cats, because of the unique structure of their spleen. Regenerative anemia, hypofunctioning spleen, spleenectomy and glucocorticoids administration are the major causes of increased Howell-Jolly bodies.35,36
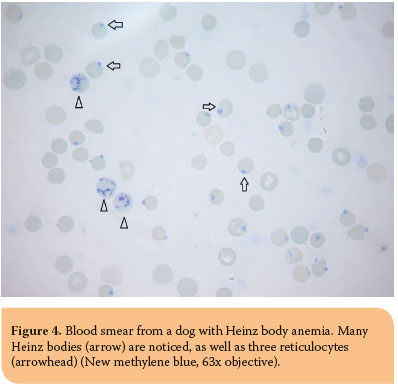
Heinz bodies are aggregates of oxidized, denatured hemoglobin which appear as small, red to pale pink, with Romanowsky-type stains, projections in erythrocyte surface. On NMB blood smears, they are stained bluish-green and are visualized much easier (Figure 4). In healthy cats, Heinz bodies may be observed in more than 5% of erythrocytes because of the decreased splenic removal and the susceptibility of cats to the denaturation of hemoglobin by endogenous oxidants.35 The etiology of Heinz bodies formation has been described thoroughly in Heinz body anemia.
 Basophilic stippling is the formation of blue-staining punctuate inclusions (in Romanowsky-stained blood smears) in erythrocytes, which represent aggregates of ribosomes and polyribosomes. Basophilic stippling must be diff erentiated from siderotic granules, which are usually seen adhered in clusters. In small animals, it occasionally occurs in regenerative anemia, whereas when observed in the absence of severe anemia, lead toxicity becomes a great possibility.35,36
Basophilic stippling is the formation of blue-staining punctuate inclusions (in Romanowsky-stained blood smears) in erythrocytes, which represent aggregates of ribosomes and polyribosomes. Basophilic stippling must be diff erentiated from siderotic granules, which are usually seen adhered in clusters. In small animals, it occasionally occurs in regenerative anemia, whereas when observed in the absence of severe anemia, lead toxicity becomes a great possibility.35,36
Siderotic inclusions (also known as Pappenheimer bodies) are iron-containing inclusions which, on Romanowsky-stained blood smears, appear as basophilic granules located in clusters near the periphery of the erythrocyte. Prussian blue staining is necessary for the confi rmation of these inclusions. Erythrocytes or reticulocytes with siderotic inclusions are called siderocytes, they are rarely if ever seen on normal blood smears and are associated with myeloproliferative diseases, hemolytic anemias, dyserythropoiesis and lead toxicity.35,36 Nucleated erythrocytes with siderotic inclusions are called sideroblasts and are linked to MDS, myeloproliferative disorders or inflammation.35,36
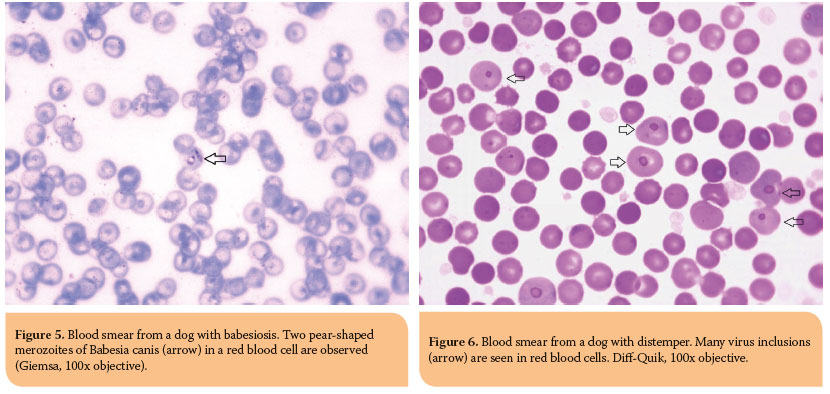
The most common erythrocyte inclusions of infectious origin are the intracellular protozoon Babesia spp., epicellular mycoplasma organisms, Mycoplasma haemofelis and Mycoplasma haemocanis, as well as distemper virus inclusions. Babesia spp. are oval to pear-shaped (usually) inclusions, which have a colorless to light blue cytoplasm and red to purple nucleus in Romanowsky-stained blood smears.35 Babesia canis is large and thus easily observed (Figure 5). On the other hand, Babesia felis and Babesia gibsoni are small and it is diffi cult to visualize them on blood smears. Hemotropic Mycoplasma spp. typically appear as cocci and occasionally as rings or rods, which are all stained in the shades of blue with Romanowsky-type stains.35 Mycoplasma haemofelis is seen individualized or in short chains, in contrast to Mycoplasma haemocanis that is seen in chains, forming various patterns.35 Distemper viral inclusions in dogs are blue-grey inclusions, variable in size and shape, formed within the erythrocyte precursors; hence, they most often occur in polychromatophilic cells, although they can be observed in erythrocytes.35 For reasons as yet unknown, the fact remains that distemper inclusions are easier to recognize with Diff -Quik staining (Figure 6).35
> References
1. Weiss D, Tvedten H. The complete blood count and bone marrow examination: general comments and selected techniques. In: Small animal clinical diagnosis by laboratory methods. Willard MD, Tvedten H (ed). 4th edn. Elsevier: St. Louis, 2004, pp. 14-37.
2. Harvey JW. Hematology procedures. In: Veterinary hematology: a diagnostic guide and color atlas. Harvey JW (ed). 1st edn. Elsevier Saunders: St. Louis, 2012, pp. 11-32.
3. Furlanello T, Tasca T, Caldin M, Carli E, Patron C, Tranquillo M, Lubas G, Solano Gallego I. Artifactual changes in canine blood following storage, detected using the ADVIA 120 hematology analyzer. Vet Clin Pathol 2006, 35(1): 42-46.
4. Stockham SL, Scott MA. Erythrocytes. In: Fundamentals of veterinary clinical pathology. Stockham SL, Scott MA (ed). 2nd edn. Blackwell Publishing: Ames, 2008, pp. 110-221.
5. Weiss D, Tvedten H. Erythrocyte Disorders. In: Small animal clinical diagnosis by laboratory methods. Willard MD, Tvedten H (ed). 4th edn. Elsevier: St. Louis, 2004, pp. 38-62.
6. Giger U. Regenerative anemias caused by blood loss or hemolysis. In: Textbook of veterinary internal medicine. Ettinger SL, Feldman EC (ed). 6th edn. Saunders Elsevier: St. Louis, 2004, pp. 1886-1907.
7. Jones Hostetter S, Andreasen CB. Anemia. In: Veterinary clinical pathology secrets. Cowell RL (ed). 1st edn. Elsevier Inc.: St. Louis, 2004, pp. 12-17.
8. Mills J. Anaemia. In: Manual of canine and feline haematology and transfusion medicine. Day MJ, Mackin A, Littlewood JD (ed). 1st edn. BSAVA: Gloucester, 2000, pp. 29-41.
9. Bush BM. Red blood cells (RBCs). In: Interpretation of laboratory results for small animal clinicians. Bush BM (ed). 1st edn. Blackwell Publishing: Oxford, 1991, pp. 31-131.
10. Gough A. Hematological fi ndings. In: Diff erential diagnosis in small animal medicine. Gough A (ed). 1st edn. Blackwell Publishing: Oxford, 2007, pp. 317-324.
11. Duval D, Giger U. Vaccine-associated immune-mediated hemolytic anemia in the dog. J Vet Intern Med 1996, 10: 290-295.
12. Desnoyers M. Anemias associated with oxidative injury. In: Schalm’s veterinary hematology. Weiss DJ, Wardrop KJ (ed). 6th edn. Blackwell Publishing Ltd.: Ames, 2010, pp. 239-245.
13. Fernadez FR, Davies AP, Teachout DJ, Krake A, Christopher MM, Perman V. Vitamin K-induced Heinz body formation in dogs. J Am Anim Hosp Assoc 1984, 20: 711-772.
14. Christopher MM. Relation of endogenous Heinz bodies to disease and anemia in cats: 120 cases (1978-1987). J Am Vet Med Assoc 1989, 194: 1089-1095.
15. Kaneko JJ. The porphyrias and porphyrinurias. In: Schalm’s veterinary hematology. Weiss DJ, Wardrop KJ (ed). 6th edn. Blackwell Publishing Ltd.: Ames, 2010, pp. 172-178.
16. Giger U. Hereditary erythrocyte enzyme abnormalities. In: Schalm’s veterinary hematology. Weiss DJ, Wardrop KJ (ed). 6th edn. Blackwell Publishing Ltd.: Ames, 2010, pp. 179-186.
17. Inaba M, Messick JB. Erythrocyte membrane defects. In: Schalm’s veterinary hematology. Weiss DJ, Wardrop KJ (ed). 6th edn. Blackwell Publishing Ltd.: Ames, 2010, pp. 187-195.
18. Jain NC. Depression or hypoproliferative anemias. In: Essentials of veterinary hematology. JainNC (ed). 1st edn, Lea & Febiger: Philadelphia, 1993, pp. 210-221.
19. Feldman BF. Nonregenerative anemia. In: Textbook of veterinary internal medicine. Ettinger SL, Feldman EC (ed). 6th edn. Saunders Elsevier: St. Louis, 2004, pp. 1908-1917.
20. Couto CG. Anemia. In: Small animal internal medicine. Nelson RW, Couto CG (ed). 4th edn. Elsevier Inc.: St. Louis, 2009, pp. 1209-1224.
21. Fry MM. Anemia of infl ammatory, neoplastic, renal, and endocrine diseases. In: Schalm’s veterinary hematology. Weiss DJ, Wardrop KJ (ed). 6th edn. Blackwell Publishing Ltd.: Ames, 2010, pp. 246-250.
22. Nangaku M, Eckardt K. Pathogenesis of renal anemia. Semin Nephrol 2006, 26: 261-268.
23. Panciera D. Conditions associated canine hypothyroidism. Vet Clin N Am Small Anim Pract 2001, 31: 935-950.
24. Weiss DJ. Pure red cell aplasia. In: Schalm’s veterinary hematology. Weiss DJ, Wardrop KJ (ed). 6th edn. Blackwell Publishing Ltd.: Ames, 2010, pp. 251-255.
25. Weiss DJ. Bone marrow pathology in dogs and cats with nonregenerative immune-mediated anemias. J Comp Pathol 2008, 138: 46-53.
26. Moore AH, Day MJ, Graham MWA. Congenital pure red blood cell aplasia (Diamond - Blackfan anaemia) in a dog. Vet Rec 1993, 132: 414-415.
27. Weiss DJ. Aplastic anemia. In: Schalm’s veterinary hematology. Weiss DJ, Wardrop KJ (ed). 6th edn. Blackwell Publishing Ltd.: Ames, 2010, pp. 256-260.
28. Weiss DJ. Aplastic anemia in the cat - clinicopathologic features and associated disease conditions 1996 - 2004. J Fel Med Surg 2006, 8: 203-206.
29. Weiss DJ. A retrospective study of the incidence and the classifi cation of bone marrow disorders in the dog at a veterinary teaching hospital. J Vet Intern Med 2006, 20: 955-961.
30. Weiss DJ. Myelodysplastic syndromes. In: Schalm’s veterinary hematology. Weiss DJ, Wardrop KJ (ed). 6th edn. Blackwell Publishing Ltd.: Ames, 2010, pp. 467-474.
31. Weiss DJ. Chronic infl ammation and secondary myelofi brosis. In: Schalm’s veterinary hematology. Weiss DJ, Wardrop KJ (ed). 6th edn. Blackwell Publishing Ltd.: Ames, 2010, pp. 112-117.
32. Weiss DJ. Iron and copper defi ciencies and disorders of iron metabolism. In: Schalm’s veterinary hematology. Weiss DJ, Wardrop KJ (ed). 6th edn. Blackwell Publishing Ltd.: Ames, 2010, pp. 167-171.
33. Randolph JF, Peterson ME, Stokol T. Erythrocytosis and polycythemia. In: Schalm’s veterinary hematology. Weiss DJ, Wardrop KJ (ed). 6th edn. Blackwell Publishing Ltd.: Ames, 2010, pp. 162-166.
34. Hasler AH. Polycythemia. In: Textbook of veterinary internal medicine. Ettinger SL, Feldman EC (ed). 6th edn. Saunders Elsevier: St. Louis, 2004, pp. 215-218.
35. Harvey JW. Evaluation of erythrocytes. In: Veterinary hematology: a diagnostic guide and color atlas. Harvey JW (ed). 1st edn. Elsevier Saunders: St. Louis, 2012, pp. 49-121.
36. Barger AM. Erythrocyte morphology. In: Schalm’s veterinary hematology. Weiss DJ, Wardrop KJ (ed). 6th edn. Blackwell Publishing Ltd.: Ames, 2010, pp. 144-161.
37. Moritz A, Fickenscher Y, Meyer K, Failing K, Weiss J. Canine and feline hematology reference values for the ADVIA 120 hematology system. Vet Clin Pathol 2004, 33(1): 32-38.



