> Abstract
Acid-base balance is an important homeostatic mechanism that focuses on maintaining a constant concentration of hydrogen ions in body fluids and it is expressed by the negative base-10 logarithm of hydrogen ion concentration (pH). The normal ranges of pH are between 7.35 and 7.45. However, when the concentration of H+ rises, a decrease of pH is observed and this condition is called acidosis. Conversely, a decrease in concentration of H+ increases pH, a condition termed alkalosis. When the above disturbances are caused by a change in the partial pressure of carbon dioxide (CO2), they are defi ned as respiratory disturbances (respiratory acidosis and respiratory alkalosis), whereas if caused by a change in the concentration of bicarbonate ions (HCO3), they are described as metabolic disturbances (metabolic acidosis and metabolic alkalosis). It is often possible that a combination of two or more disorders may occur (mixed disorders). Typically, the diagnosis of acid-base disorders is based on the measurement of pH, partial pressure of CO2 and the concentration of HCO3 in an arterial blood sample. In cases where the above control is not possible, the diagnosis or suspicion of occurrence of an acid-base disorder is established on the fi ndings of the clinical examination and electronic monitoring of vital functions. Knowledge of the mechanism by which a disease can cause an acid-base disorder is of signifi cant assistance to the veterinarian. The detection of any acid-base disturbance leads to early and intensive treatment which aims to eliminate the cause of primary disease.
> General
Acids and bases are constantly added to body fl uids, either because they are ingested with food or because they are produced in the body as a by-product of the metabolism. Acid-base balance, i.e. the balance between acids and bases, constitutes an important homeostatic mechanism. Maintenance of this balance focuses essentially on the regulation of hydrogen ion (H+) concentration in body fluids. Even a small deviation of the concentration from normal values can cause signifi cant changes in cell function, as in the ionization of drugs, thus aff ecting their pharmacokinetics and pharmacodynamics.1 For practical reasons, the concentration of H+ is expressed according to their negative base-10 logarithm and is called pH.1,2 Normally, the pH value of blood plasma ranges from 7.35 to 7.45.1 This value indicates remarkable stability despite the continuous acid production, and this is due to the body’s pH resistivity with the activity of intracellular and extracellular regulatory systems such as proteins, haemoglobin etc.1-3 Subsequently, and for the same purpose, an acid-base disorder is counterbalanced, i.e. the body causes partial neutralization of the disorder, mainly through the respiratory and urinary system, attenuating the change of pH.1,2
However, apart from the concentration of H+, an important role in acid-base balance is played by water, carbon dioxide and bicarbonate ions, which are connected to each other by the following equation:
H2O + CO2 H2CO3 HCO3 - + H+
(equation 1)
In the presence of water, carbon dioxide is converted to carbonic acid, a reaction which is catalyzed by carbonic anhydrase and is bidirectional. The dissociation of carbonic acid to bicarbonate ions and hydrogenions is also bidirectional. It is obvious that the elimination of HCO3 - (e.g. diarrhoea) causes a relative increase in H+ and therefore acidosis (pH < 7.35); CO2 elimination (e.g. tachypnoea) causes a relative reduction in H+ and therefore alkalosis (pH > 7.45); the increase of H+ (e.g. lactic acid) causes acidosis (pH < 7.35) and so on.1,2,4 Generally, simple acid-base balance disorders caused by changes in CO2 partial pressure originate from the respiratory system (respiratory acidosis, respiratory alkalosis), while those caused by a change in HCO3 - and H+ are metabolic in origin (metabolic acidosis, metabolic alkalosis). Critically ill animals sometimes develop mixed acid-base disorders.1,2
> Diagnosis of Acid-Base Disorders
The diagnosis of simple acid-base disorders is relatively easy in cases where it is possible to measure the pH, the partial pressure of CO2 and the concentration of HCO3 - in an arterial blood sample.2,5 However, when this is not possible, the veterinarian is able to identify and treat them based only on the clinical signs and symptoms of the disease that causes them or with information based on the control of vital functions by electronic or other means. The awareness and concern of the clinician for a coexisting and potentially serious acid-base disorder, as well as an understanding of the primary disease mechanism causing these disorders is of valuable help.
1. Respiratory acidosis
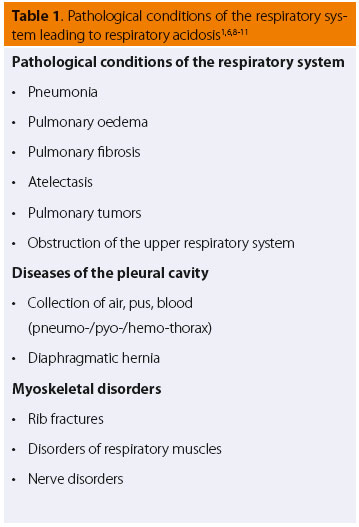
Pathological Conditions and clinical findings of the Respiratory System
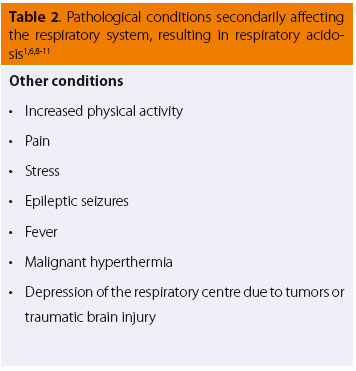
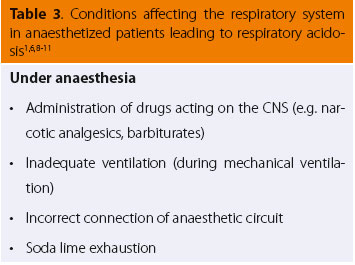
Many cases of pathological conditions of the respiratory system (Table 1) or those that secondarily affect the respiratory system (Table 2) commonly share certain clinical findings. These include changes in the respiratory rate and tidal volume (indicated by expansion of the chest wall or the anaesthetic circuit bag of intubated patients connected to the anaesthetic machine).6,7 (Table 3) The increase in respiratory rate is often accompanied by a reduction in tidal volume, which can lead to a decrease in respiratory minute volume. In severe cases, cyanosis of the visible mucosal may be observed, which is a clinical sign suggesting that impaired lung function is accompanied by inadequate tissue oxygenation.
 Findings from the electronic monitoring
Findings from the electronic monitoring
When monitoring of vital functions by electronic means is viable, as for example in anaesthetized animals, the veterinarian may observe an increase in the end-tidal CO2 (ETCO2)1,6,7,11 and in some cases a reduction of haemoglobin oxygen saturation (SpO2),12 especially when the animal is breathing atmospheric air. It should be noted that normally when the patient breathes atmospheric air, haemoglobin saturation should be above 92% and when breathing 100% O2, it should be over 98%, while in the clinical practice, a percentage less than 85% (hypoxaemia) requires immediate correction.
Determination of acid-base balance
When the veterinarian detects the above symptoms combined with any of the aforementioned situations (pathological or not), he should suspect that the lungs can not suffi ciently eliminate the CO2 produced by the body, thereby causing an increase in the partial pressure of CO2 in the blood, a condition known as hypercapnia. Hypercapnia causes acidosis, which due to its origin is characterized as respiratory acidosis. Meanwhile, the O2 uptake may not be suffi cient for the needs of the body.4,11 In acute cases, the patient is often at risk due to hypoxaemia, before hypercapnia becomes severe and life-threatening. It should be noted that abrupt termination of oxygenation results in death after about four minutes, while the increase in non-eliminated CΟ2 at life-threatening levels takes about 10-15 minutes to develop.6,13
Clinical Signs from other organs/systems
In addition to the clinical signs resulting from the respiratory system, acidosis may cause the cardiovascular system to also display observable signs.6,10 More specifically, tachycardia1 and arrhythmias (including ventricular fibrillation) can occur due to the increase in the tone of the sympathetic system and hypoxaemia when it coexists.6,9,14,15 Moreover, according to experiments carried out in dogs, the changes observed primarily in blood pressure are small since the increase in heart rate is compensated by the decrease in myocardial contractility and systemic vascular resistance.6,16
Depending on the extent of hypercapnia, production rate and coexisting hypoxaemia, dilation of brain blood vessels can be observed, followed by an increased cerebral blood flow and increased intracranial pressure.6,17-19 Thus, the observed clinical signs include restlessness, disorientation, nystagmus, and even coma (in awake animals).6,8,10,20,21
Confirmation of the presence of respiratory acidosis (arterial blood gas sample analysis)
Consequently, in cases of reduced tidal volume with or without cyanosis, and possibly disorders of the cardiovascular system, it is likely that an arterial blood gas sample analysis would reveal a fall in pH, an increase (small or large) in the partial pressure of CO2 (depending on the severity of the disease), and a reduction in the partial pressure of O2. As concerns the concentration of bicarbonate ions (HCO3 -), this is normal in acute disorders since they constitute a compensatory mechanism of the body that needs time to be activated.1,5
Treatment of respiratory acidosis
- Initial treatment
The first step in the treatment of respiratory acidosis is the elimination of the cause, which in most cases is sufficient. For example, in cases of pleural eff usion, thoracentesis is required, while the administration of drugs that suppress the respiratory system should be discontinued (if possible) or modified.6,11
- Oxygen administration
The administration of oxygen in respiratory acidosis is beneficial since it is often accompanied by varying degrees of hypoxaemia.11 In acute and severe conditions accompanied by cyanosis, it is necessary to administer oxygen or intubate the trachea and apply artificial ventilation until the causing factor is treated.6,22
-Administration of alkalinizing solutions
The administration of sodium bicarbonate or other alkalinizing agents is contraindicated as it leads to an increase of CO2 in the blood (equation 1), i.e. worsening of the existing hypercapnia and respiratory acidosis.6,23,24
2. Respiratory alkalosis
Pathological conditions and clinical signs
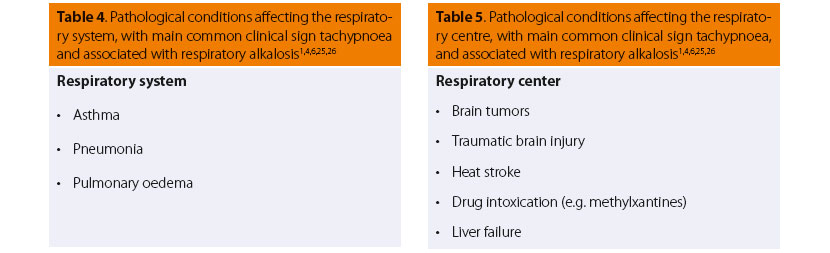
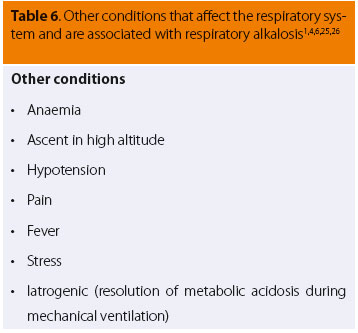
In many cases, in conditions directly or indirectly aff ecting the respiratory system (Table 4) or brainstem respiratory centres (Table 5), and also in other situations such as those listed in Table 6, the main common clinical symptom is tachypnoea.6,7 The other clinical signs are mainly due to the primary disease, such as vomiting or diarrhoea in cases of methylxanthines intoxication, pale mucous membranes in cases of anaemia, etc.
 Findings from the electronic monitoring
Findings from the electronic monitoring
When there is the possibility of monitoring vital functions electronically, the veterinarian will observe a decrease of end-tidal CO2 (ETCO2)25 while in cases of hypoxaemia, there is a characteristic decrease of haemoglobin saturation.
Determination of acid-base disorder
Thus, the combination of the aforementioned clinical signs with one of the above situations (pathological or not) suggests that tachypnoea, i.e. the increase in lung ventilation, or otherwise hyperventilation, increases elimination of CO2 during the breathing process, leading to reduction in its partial pressure in the blood, i.e. hypocapnia, which results in respiratory alkalosis.
In veterinary medicine, other than tachypnoea, no other clinical signs specifically associated with alkalosis have been described, perhaps due to the efficient, metabolic compensatory response of the body. Conversely, according to studies conducted in humans, there may occur confusion, restlessness, disorientation, convulsions and myoclonus due to increased neuromuscular excitability and reduced brain blood flow.1,6,20,27 Furthermore, because of hypercapnia, and hence the absence of the vasodilating eff ect of CO2, peripheral vasoconstriction is observed and hypertension may possibly be present.1,6 Finally, although hypocapnia has little eff ect on the myocardium, it predisposes to supraventricular and ventricular arrhythmias, particularly in patients with pre-existing cardiovascular problems.6,20
Confirmation of the presence of respiratory alkalosis (arterial blood gas sample analysis)
Respiratory alkalosis is an acid-base disorder that is difficult to identify because it accompanies diseases with predominant clinical signs; hence, alkalosis is not easily diagnosed as a distinct disease.4,6
The confirmation of respiratory alkalosis is based on the combination of the results of physical examination and those from the arterial blood gas samples, namely increased pH and decreased partial pressure of CO2.5,6,25,28 In most patients, haemoglobin saturation (SpO2) is within normal limits, except for those cases where low SpO2 in itself is a cause of tachypnoea and hypocapnia. The observed changes of bicarbonate ion concentration in the blood are compensatory. In acute respiratory alkalosis, no changes in their concentration are perceived if no other infl uencing factor coexists as it takes time for the compensatory mechanism of the kidneys to activate.5,6,25
Treatment of respiratory alkalosis
- Initial treatment
Most important is the identifi cation and correction of the underlying cause.1,4,6,7,25,28 Normally, no other treatment is required, apart from relieving the underlying cause that led to hyperventilation of the lungs and thus the respiratory disorder (e.g. discontinuation of methylxanthine administration, when the disorder is due to toxicosis of these drugs, or analgesic drugs when the disorder is a result of nociceptive stimulus etc.).
- Correction of the observed hypocapnia
The observed hypocapnia does not usually reach dangerous levels; hence, no eff ort for correction of this disorder is necessary apart from special cases, such as a traumatic brain injury where it can lead to excessive vasoconstriction and ischaemia of regions of the brain or in intubated patients receiving artifi cial ventilation (iatrogenic hyperventilation) where reduction of ventilation is demanded for the correction of the disorder.
-Oxygen administration and hypoxaemia
When hypoxaemia coexists, the administration of Ο2 is mandatory and also serves to reduce respiratory alkalosis by decreasing the induced hyperventilation.
- Administration of analgesics and sedatives etc.
Finally, for the treatment of disorders due to “physiological” factors, such as situations of fear, pain or stress, the administration of analgesic or sedative drugs has proven to be a satisfactory therapeutic measure (e.g. administration of analgesia or/and sedation postoperatively).25
3. Metabolic acidosis
Pathological conditions and clinical signs from the respiratory system
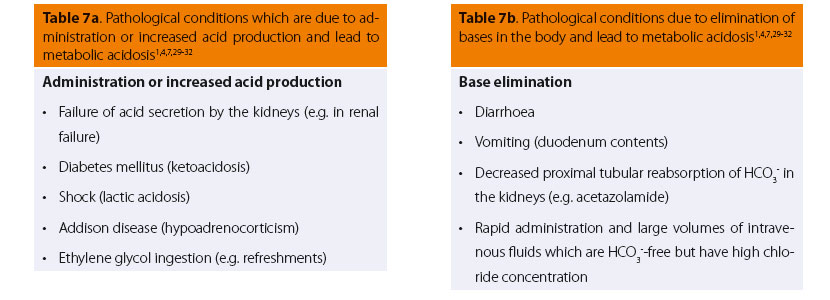
In many cases of pathological disorders (Tables 7a and 7b) that seriously affect the general condition of the patient, such as diarrhoea, shock with impaired tissue oxygenation and lactic acid production (lactic acidosis), uremic crisis, and ketoacidotic diabetes mellitus,1,29,30 a common clinical symptom emerges from the respiratory system: ventilation of the lungs increases markedly. In humans, this type of breathing is called Kussmaul. This breathing pattern is characterised by prolonged respiratory movements with an extreme increase in range, even when the patient is at rest. Apart from the type of breathing, the increase in ventilation is also evident from the fall in end-tidal CO2 (ETCO2) during electronic monitoring.7,30
Clinical Signs from other organs/systems
Apart from the specifi c breathing pattern, there are some common clinical signs. Specifically, the signs observed by the cardiovascular system include reduction of the blood pressure, and/or presence of ventricular arrhythmia.1,7,15,30,31,33 Usually, there are no signs from the central nervous system (CNS), unless the primary pathological disorder progresses without treatment, hence a semi-comatose state may be observed.1,7,30,34
Determination of acid-base disorder
The above signs in patients without a respiratory problem, in conjunction with the patient’s history and aetiological diagnosis, lead the clinician to suspect metabolic acidosis.
Metabolic acidosis occurs when the kidneys are not able to excrete the H+ that accumulate in the body in cases of overproduction of H+ or when there are large losses of HCO3 -. Thus, a reduction in blood pH is observed and the concentration of HCO3 - in blood plasma also decreases because when acids in the body increase, an amount of H+ concentrations are neutralized by ΗCO3 -, thereby causing the concentration of the latter in the blood to decrease.
Confirmation of the presence of metabolic acidosis (arterial blood gas sample analysis)
When arterial blood gas analysis is possible, decrease in pH, reduction of bicarbonates and decrease in Pco2 are expected, the latter due to compensatory hyperventilation.5,7,30,31,35,36 It is worth noting that hyperkalaemia is not a consistent fi nding in metabolic acidosis; it is observed in cases where acidosis is due to inorganic acids or where other causes of hyperkalaemia, such as insulin defi ciency, may coexist.30,34,37
Treatment of metabolic acidosis
- initial treatment
Metabolic acidosis is the most common disorder of acid-base balance. The recognition and early correction of this disorder is important because of the pathophysiological changes that may be caused by the decreased pH of arterial blood.31
The aim of the therapeutic approach is to treat the primary disease urgently and effectively, correct electrolyte imbalances and finally maintain efficient kidney function and support kidney perfusion. If all these objectives are achieved, the homeostatic mechanisms of the body will restore the metabolic disorder, usually without any further action required by the clinician.4,7,30-32
- Administration of crystalloid fluids
When the metabolic disorder is due to loss of bases or where the addition or increase of acids is observed, in addition to treating the primary cause whenever possible (e.g. administration of insulin in cases of diabetes mellitus), the concurrent aggressive administration of intravenous crystalloids (e.g. Lactated Ringer’s solution) not only constitutes part of the primary disease treatment, but it will also accelerate the correction of metabolic acidosis.4
- Administration of alkalinizing agents
Alkalinizing agents can be administered, such as sodium bicarbonate (NaHCO3), in cases where acidosis is proven to be severe. However, they should be administered very carefully in order to avoid iatrogenic complications, like metabolic alkalosis, hypernatraemia and paradoxical (respiratory) acidosis when the produced CO2 (equation 1) is not suffi ciently eliminated by the lungs.4,7,30,31
4. Metabolic alkalosis
Pathological conditions, clinical signs and fi ndings from monitoring
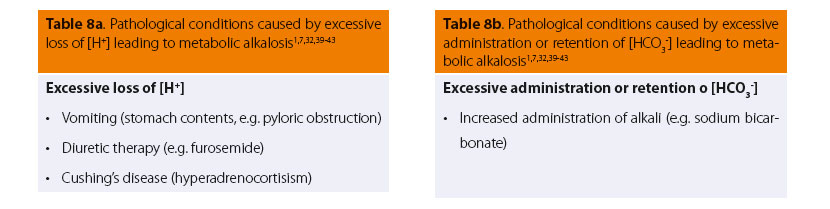
In certain pathological conditions, such as vomiting after pyloric obstruction or administration of diuretic drugs (mainly loop diuretics), etc. (Tables 8a and 8b), apart from the signs of each primary disease, certain other signs are common, such as lethargy or conversely restlessness, disorientation, ataxia, which in rare cases can progress to muscle contractions, seizures or coma.7,30,38 Other clinical signs observed include hypotension (due to loss of intravascular volume and peripheral vasodilation), muscle weakness, arrhythmias (due to the disorder of electrical conductivity of myocardial cells), disturbances of gastrointestinal motility (e.g. ileus) and disorders of renal function (decreased ability of the kidneys to reabsorb water).
Laboratory findings
Evaluation of the concentration of electrolytes in blood samples reveals hypokalaemia, which frequently coexists with hypovolaemia and is responsible for many signs described above. However, the muscle contractions observed may be due to the reduced concentration of Ca+2 level in the blood.30
Determination of acid-base disorder
These signs, combined with the patient’s history (e.g. vomiting, administration of diuretic drugs, hyperadrenocorticism or hypokalaemia) and fi nal diagnosis, are important clues that enable the clinician to establish the diagnosis of metabolic alkalosis.30
Metabolic alkalosis is a disorder that occurs when the bases are increasing in the body or the acids are decreasing. Losses of Η+ from the body, such as vomiting of gastric fluids or base uptake, an alkalinizing agent or increased HCO3 - due to its inadequate elimination (e.g. renal failure), are conditions which may lead to metabolic alkalosis.1
Confirmation of the presence of metabolic alkalosis (arterial blood gas sample analysis)
When the evaluation of arterial blood gas samples is possible, the results are increased pH and elevated concentration of bicarbonates; with regard to partial pressure of CO2, a slight increase is observed; and as a result of the metabolic disorder, the compensatory respiratory mechanism is activated and hypoventilation is induced, hence decreasing the respiratory rate and volume of breathing. If the animal is breathing room air (21% O2), hypoventilation will not exacerbate as the concurrent reduction of the partial pressure of O2 in the blood will stimulate the respiratory centre, thus resulting in elimination of CO2 which tends to accumulate.30,36,44 Finally, in electrolytic analysis, the results very often reveal hypokalaemia, hypocalcaemia and hypochloraemia, as already mentioned.
Treatment of metabolic alkalosis
- Initial treatment
The removal of the primary cause and restoration of circulating blood volume and electrolyte balance, where needed, are the three points of therapeutic approach.4,44 The combination of an electrolytic solution rich in chlorine (e.g. NaCl 0.9%,) without precursor molecules of bicarbonates, in combination with KCI, is the most suitable crystalloid fl uid for treating both alkalosis and hypokalaemia and for the restoration of electrolyte neutrality.30,32 The restoration of chlorine levels in the blood can aid in the excretion of HCO3 -.7,30 Although it may take some days for the restoration of acid-base balance, in almost all cases these measures are suffi cient.30,32
- Administration of H2-blockers
The administration of H2-blockers (e.g. cimetidine, ranitidine, famotidine) may be used as adjunctive therapy when gastric losses increase. Furthermore, when administration of diuretic agents cannot be interrupted, H2-blockers can be used instead of or with loop diuretics, spironolactone or amiloride, which limit the alkalinizing activity of the first by maintaining chloride ions.7,30
- Avoidance of oxygen administration
Finally, when patients who suffer from chronic respiratory diseases with chronic hypoxaemia and hypercapnia develop metabolic alkalosis, they are more likely to display further reduction in ventilation and exacerbated hypoxaemia. However, in these patients it is suggested that oxygen therapy should be avoided where possible since acute correction of chronic hypoxaemia may result in further reduction in ventilation and worsening hypercapnia.30
> Conclusion
Although acid-base disorders constitute an important problem in clinical practice, the veterinarian has limited means to approach them without special equipment, such as a blood gas analyzer. However, if the veterinarian is concerned for the coexistence of an acid-base disorder and also has the knowledge of how a primary disease may cause this imbalance, then based only on clinical signs, he/she may conclude the existence of a simple acid-base disorder which explains the severity of the signs, and thus proceed to a more direct and intensive treatment of the primary disease.
> References:
1. Σμοκοβίτης Αθ, Φυσιολογία των κατοικιδίων ζώων, 5η έκδοση, εκδό- σεις Κυριακίδης : Θεσσαλονίκη, 2007, σελ. 920-937.
2. Johnson AR, de Morais HA, Introduction to acid-base disorders. In : Fluid, electrolyte and acid-base disorders in small animal clinical practice, DiBartola SP editor, 4th edition, Elsevier Saunders: St. Louis, Missouri, 2012, pp. 231-251.
3. Madias NE, Cohen JJ, Acid-base chemistry and buff ering. In : Acid- base, Cohen JJ, Kassirer JP, editors, Little Brown& Co: Boston, 1982, pp. 13- 17.
4. Silverstein DC, Hopper K, Small animal critical care medicine, 2nd edition, Elsevier Saunders: St. Louis, Missouri, 2015, pp. 289-295.
5. Mensack S, Analyzing blood gases. In: Critical care: quick look series in veterinary medicine, Murtaugh RJ, TetonNewMedia: Wyoming, 2002, pp. 28-29.
6. Johnson AR, de Morais HA, Respiratory acid-base disorders. In: Fluid, electrolyte and acid-base disorders in small animal clinical practice, DiBartola SP editor, 4th edition, Elsevier Saunders: St. Louis, Missouri, 2012, pp. 287-301.
7. Κωστάκης Χ, Καζάκος Γ, Οξεοβασικές διαταραχές στην κλινική πράξη: είναι πάντα απαραίτητος ο αναλυτής αερίων αίματος; 4ο forum κτηνιατρι- κής ζώων συντροφιάς, ΕΛΕΚΖΣ, Θεσσαλονίκη, 2013.
8. Madias NE, Cohen JJ, Respiratory acidosis. In: Acid-base, Cohen JJ, Kassirer JP, editors, Little Brown& Co: Boston, 1982, pp. 307-48.
9. Epstein SK, Singh N, Respiratory acidosis, Respir Care, 2001, 46: 366- 383.
10. Wall RE, Respiratory acid-base disorders, Vet Clin North Am Small Anim Pract, 2001 (Nov), 31(6): 1355-1367. 11. Mensack S, Respiratory acidosis. In: Critical care: quick look series in veterinary medicine, Murtaugh RJ, TetonNewMedia: Wyoming, 2002, pp. 32-33.
12. Greene SA, Veterinary anaesthesia and pain management secrets, Hanley & Belfus, inc.: Philadelphia, 2002, pp. 121-126.
13. Madias NE, Adrogué HJ, Cross-talk between two organs: how the kidney responds to disruption of acid-base balance by the lung, Nephron Physiol, 2003, 93(3): 61-66.
14. Kerber RE, Pandian NG, Hoyt R, Jensen SR, Koyanagi S, Grayzel J, Kieso R, Eff ect of ischemia, hypertrophy, hypoxia, acidosis, and alkalosis on canine defi brillation, Am J Physiol, 1983 (June), 244(6): H825-831.
15. Orchard CH, Kentish JC, Eff ects of changes of pH on the contractile function of cardiac muscle, Am J Physiol, 1990 (June), 258(6 pt: 1): C967- 981.
16. Walley KR, Lewis TH, Wood LD, Acute respiratory acidosis decreases left ventricular contractility but increases cardiac output in dogs, Circ Res, 1990 (Sept), 67(3): 628-635.
17. Alberti E, Hoyer S, Hamer J, Stoeckel H, Packschiess P, Weinhardt E, The eff ect of cardon dioxide on cerebral blood fl ow and cerebral metabolism in dogs, Br J Anaesth, 1975 (Sept), 47(9): 941-947.
18. Kontos HA, Raper AJ, Patterson JL, Analysis of vasoactivity of local pH, pCO₂, and bicarbonate on pial vessels, Stroke, 1977 (May-June), 8(3): 358-360.
19. Williams G, Roberts PA, Smith S, Stevens FA, Tompkins P, Arancibia C, Pollay M, The eff ect of apnea on brain compliance and intracranial pressure, Neurosurgery, 1991 (Aug), 29(2): 242-246.
20. Andogué HJ, Madias NE., Management of life-threatening acid-base disorders (two parts), N Engl J Med, 1998 (Jan) 1.338(1): 26-34.
21. Nunn JF, Changes in the carbon dioxide tension. In: Applied respiratory physiology, Nunn JF, 5th edition, Butterworth-Heinemann: Edinburgh, 2000, pp. 460-471.
22. Bateman SW, Ventilating the lung injured patient: what’s new? In:Proceedings of the American College of Veterinary Surgeons Symposium, Chicago, 2001, pp. 562-565.
23. Arvidsson S, Häggendal E, Winsö I, Infl uence on cerebral blood fl ow of infusion of sodium bicarbonate during respiratory acidosis and alkalosis in the dog, Acta Anaesthesiol Scand, 1981 (Apr), 25(2): 146-152.
24. Nishikawa T, Acute haemodynamic eff ects of sodium bicarbonate in canine respiratory and metabolic acidosis, Br J Anaesth, 1993 (Feb), 70(2): 196-200.
25. Mensack S, Respiratory alkalosis. In: Critical care quick look series in veterinary medicine, Murtaugh RJ, TetonNewMedia: Wyoming, 2002, pp. 36-37.
26. Lumb AB, Control of breathing. In: Applied respiratory physiology, Nunn JF, 7th edition, Churchill Livingston: Philadelphia, 2010, pp. 61-82.
27. Gennari FJ, Kassirer JP, Respiratory alkalosis. In: Acid-base, Cohen JJ, Kassirer JP editors, Little Brown& Co.: Boston, 1982, pp. 349-376.
28. Norkus CL, Veterinary Technician’s Manual for Small Animal Emergency and Critical Care, Wiley-Blackwell publication, West Sussex, 2012, pp. 433-463.
29. Hopper K, Epstein SE, Incidence, Nature, and Etiology of Metabolic Acidosis in Dogs and Cats, J Vet Intern Med, 2012 (Sept-Oct), 26(5): 1107- 1114.
30. DiBartola SP, Metabolic acid-base disorders. In: Fluid, electrolyte and acid-base disorders in small animal clinical practice, DiBartola SP editor, 4th edition, Elsevier Saunders: St. Louis, Missouri, 2012, pp. 253-286.
31. Mensack S., Metabolic acidosis. In: Critical care: quick look series in veterinary medicine, Murtaugh RJ, TetonNewMedia: Wyoming, 2002, pp. 30-31.
32. Dethioux F., Handbook for the veterinary practitioner: emergency medicine, Selected topics in canine and feline emergency medicine, Royal Canin, volume 1, pp. 122-136.
33. Adrogue HJ, Brensilver J, Madias NE, Changes in the plasma anion gap during chronic metabolic acid-base disturbances, Am J Physiol, 1978 (Oct), 235(4): F291-297.
34. Adrogue HJ, Madias NE, Changes in plasma potassium concentration during acid-base disturbances, Am J Med, 1981 (Sept), 71(3): 456-467.
35. Cornelius LM, Rawlings CA, Arterial blood gas and acid base values in dogs with various diseases and signs of disease, J Am Vet Med Assoc, 1981 (May), 178(9): 992-995.
36. Matthews KA., Veterinary emergency and critical care manual, Lifelearn inc.: Canada, 2006, pp. 406-410.
37. Madias NE, Lactic acidosis, Kidney Int., 1986, 29: 752.
38. Harrington JT, Kassirer JP, Metabolic alkalosis. In: Acid-base, Cohen JJ, Kassirer JP editors, Little Brown& Co: Boston, 1982, p. 240.
39. Grantham JJ, Schloerb PR., Acute subtraction alkalosis from gastric juice loss in dogs, Am J Physiol, 1964 (Sept), 207: 619-626.
40. Muir WW, Acid-base and electrolyte disturbances in dogs with gastric dilatation-volvulus, J Am Vet Med Association, 1982 (Aug) 1, 181(3): 229- 231.
41. Robinson EP, Hardy RM, Clinical diagnosis and treatment of alkalemia in dogs: 20 cases (1982-1984), J Am Vet Association, 1988 (Apr) 1, 192(7): 943-949.
42. Boag AK, Coe RJ, Martinez TA, Hughes D, Acid-base and electrolyte abnormalities in dogs with gastrointestinal foreign bodies, J Vet Intern Med, 2005 (Nov-Dec), 19(6): 816-821.
43. Y.-S. Ha, Hopper K, Epstein SE, Incidence, nature and etiology of metabolic alkalosis in dogs and cats, J Vet Intern Med, 2013 (Jul-Aug), 27(4): 847–853.
44. Rose BD, Post TW, Metabolic alkalosis. In: Clinical physiology of acid- base and electrolyte disorders, 5th edition, McGraw-Hill: New York, 2001, pp. 551-577.



