> Abstract
The recent discovery of the existence in the retina of a third group of photosensitive cells, other than cones and rods, which have the capacity of stimulating the pupillary light reflex, has changed our knowledge of how the iris reacts to different wavelengths of light and introduced the concept of chromatic pupillary light reflex in eye examination. This is a test whereby the pupillary response is stimulated not by monochromatic white light, but successively by red and blue light, allowing the selective stimulation of photoreceptors. The chromatic pupillary light reflex is particularly useful in the diagnosis of sudden acquired retinal degeneration, progressive retinal atrophy, chorioretinopathies, retinal detachment, glaucoma, disorders of the optic nerve and optic chiasm and certain brain diseases that cause blindness.
> Pupillary light reflex: existing knowledge
According to the classical anatomical description, the retina has two types of photoreceptors1 : rods, which are more numerous and responsible for dim-light vision, and cones, which function in daylight and are responsible for recognising colours.2 Rods contain the photosensitive pigment rhodopsin that presents maximal sensitivity at 508nm (blue/green spectrum). The canine retina has two types of cones containing the photosensitive pigment opsin. The first type presents maximal sensitivity at 555nm (L/M opsin - green spectrum) and the second at 430nm (S opsin - violet spectrum).3,4,5
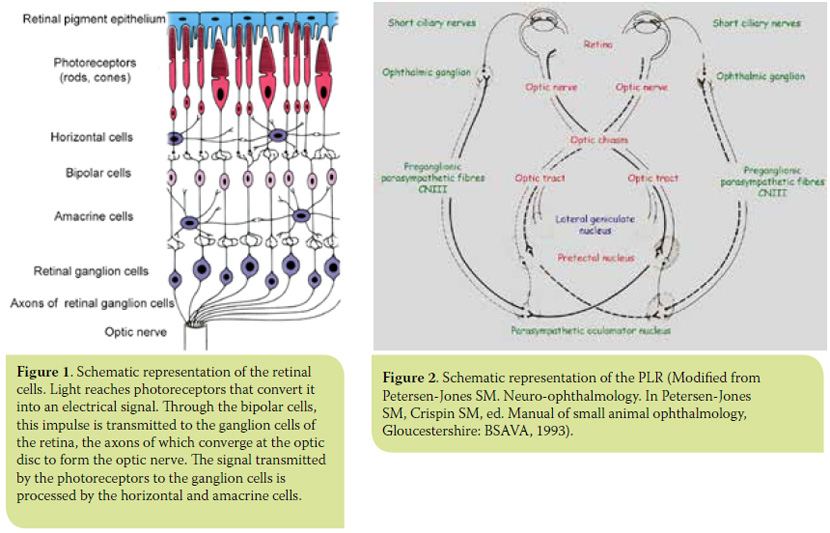
Cones and rods transduce light energy into an electrical signal that is transmitted to the ganglion cells of the retina through the bipolar cells. Two other cell types in the retina, the horizontal and amacrine cells, are involved in the processing of the impulse transmitted from the photoreceptors to the retinal ganglion cells (RGCs). Axons of the RGCs constitute the optic nerve fibres (Figure 1). The optic nerve fibres, after decussating at the optic chiasm, form the optic tract and are then divided into two bundles. The majority (80-90%) of RGC axons, which serve the function of vision, synapse in the lateral geniculate nucleus (LGN). The axons of the LGN cells (optic radiation) end in the posterior occipital lobe of the cortex, the visual cortex. A smaller subset (10-20%) of RGC axons, which serves the pupillary light reflex (PLR), synapse in the pretectal nucleus from which there are neural connections with the ipsilateral and contralateral parasympathetic oculomotor nucleus (PON) providing afferent input to the PLR2,6 (Figure 2).
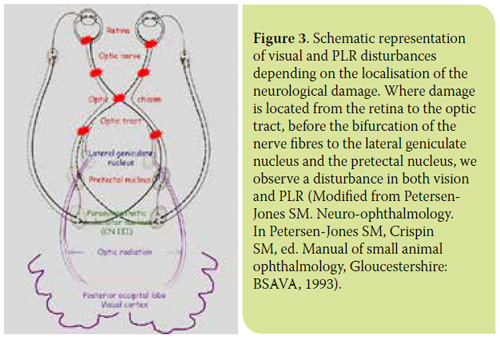
PLR concerns the contraction of the sphincter muscle of the iris and constriction of the pupil following light stimulation of the eye. PLR is considered normal when it is rapid, complete (pupil diameter 5 mm) and stable. However, the assessment of PLR should take into account certain factors that cause mydriasis and affect it, such as the animal’s stress during examination, iris atrophy in some older animals and administration of certain drugs.7 Direct PLR concerns miosis induced after stimulation of the ipsilateral eye, while indirect PLR miosis induced after stimulation of the contralateral eye.
PLR is controlled by the autonomic parasympathetic system and its assessment is an integral part of the ophthalmic and neurological examination. PLR examination uses a focal monochromatic white light source, in order to test the integrity of the reflex arc, i.e. the retina, optic nerve, optic chiasm, optic tract, pretectal nucleus, PON, preganglionic parasympathetic fibres of the oculomotor nerve, ophthalmic ganglion, and short ciliary nerves (Figure 2). PLR does not test visual function. Thus, depending on the location of the lesion, there are some cases where loss of PLR may be accompanied by loss of vision, and others of blind animals presenting normal PLR8-11 (Figures 3-5).
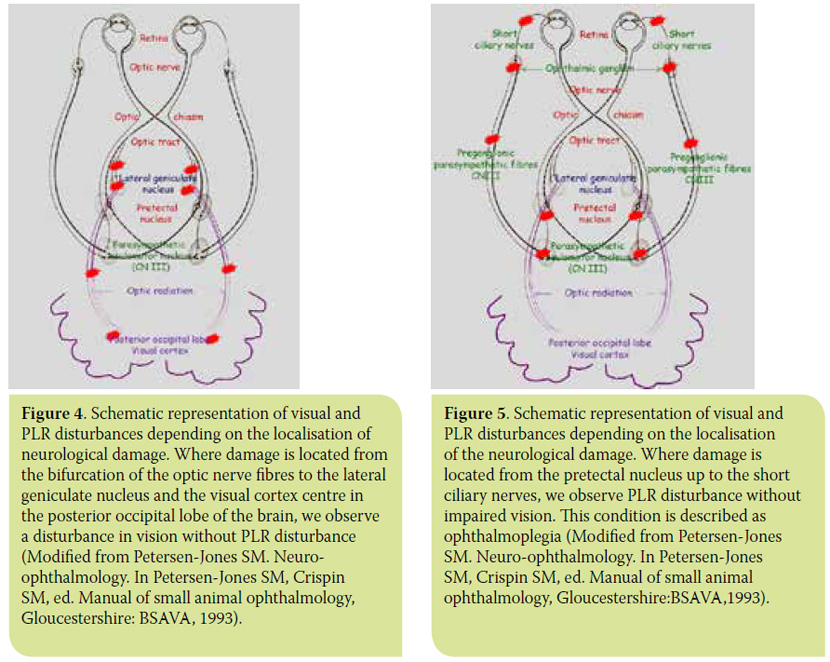
It has been observed that some patients suffering from blindness due to certain retinal diseases present positive PLR (though delayed and/or incomplete and unstable in most cases). Until recently, this was attributed to the small number of retinal photoreceptors that remain functional and are able to stimulate PLR. Traditionally, white light sources are used for evaluation of PLR. As PLR observation is used in assessing diseases that can affect the retina, optic nerve and anterior visual pathways, the presence of afferent deficits after white light stimulation does not differentiate whether problems are present in the retina or at the level of the optic nerve and/or brain.12
> Latest data: melanopsin and intrinsically photosensitive retinal ganglion cells
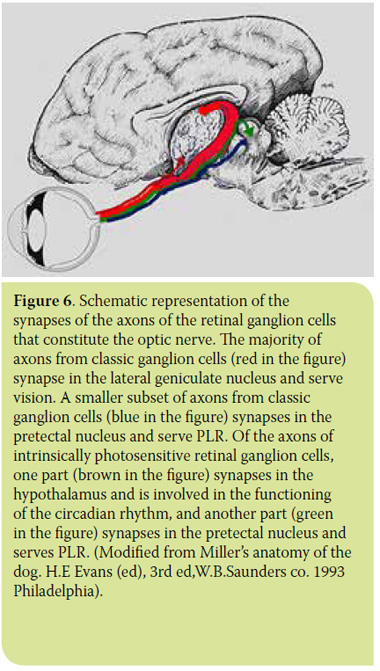 In 1923, Clyde Keeler observed that members of a colony of genetically modified mice, whose retina lacked cones and rods, presented normal PLR. In 1927, his research led to the conclusion that besides cones and rods, there is a third group of photoreceptors in the retina - most likely certain ganglion cells - capable of stimulating PLR.13 Unfortunately, Keeler’s discovery was not accepted by the scientific community. Research in this direction was interrupted and was to continue much later in the 1990s, eventually leading to the discovery in the early 2000s of the existence of a new photopigment and a third group of photoreceptors in the retina. We now know that in addition to rhodopsin and opsin, the retina also has another photosensitive pigment, melanopsin, which exhibits maximal sensitivity at 480nm (blue spectrum).14-18 Melanopsin is contained in certain RGCs, called intrinsically photosensitive retinal ganglion cells (ipRGCs).16,19-21 These cells represent 1-3% of all RGCs and owe their name to their capacity for being stimulated by light, irrespective of the stimulation of classic photoreceptors (rods and cones). The neural connections of these cells are of particular interest. The axons of ipRGCs do not synapse in the LGN but in the suprachiasmatic nucleus of the hypothalamus, pineal gland and PON (Figure 6). Consequently, ipRGCs do not participate in the visual function, but in the functions of the circadian rhythm, of the sleep/wake pattern and finally in PLR and the photopic blink response.22-28
In 1923, Clyde Keeler observed that members of a colony of genetically modified mice, whose retina lacked cones and rods, presented normal PLR. In 1927, his research led to the conclusion that besides cones and rods, there is a third group of photoreceptors in the retina - most likely certain ganglion cells - capable of stimulating PLR.13 Unfortunately, Keeler’s discovery was not accepted by the scientific community. Research in this direction was interrupted and was to continue much later in the 1990s, eventually leading to the discovery in the early 2000s of the existence of a new photopigment and a third group of photoreceptors in the retina. We now know that in addition to rhodopsin and opsin, the retina also has another photosensitive pigment, melanopsin, which exhibits maximal sensitivity at 480nm (blue spectrum).14-18 Melanopsin is contained in certain RGCs, called intrinsically photosensitive retinal ganglion cells (ipRGCs).16,19-21 These cells represent 1-3% of all RGCs and owe their name to their capacity for being stimulated by light, irrespective of the stimulation of classic photoreceptors (rods and cones). The neural connections of these cells are of particular interest. The axons of ipRGCs do not synapse in the LGN but in the suprachiasmatic nucleus of the hypothalamus, pineal gland and PON (Figure 6). Consequently, ipRGCs do not participate in the visual function, but in the functions of the circadian rhythm, of the sleep/wake pattern and finally in PLR and the photopic blink response.22-28
> Chromatic pupillary light reflex
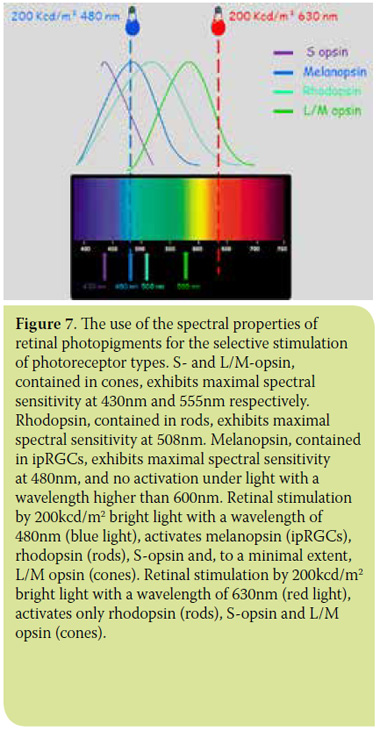 Examining the spectral properties of photosensitive pigments in the canine retina, we observe that the use of bright light, at a wavelength of 630nm (red spectrum), stimulates only the rods and cone types containing L/M opsin. The use of bright light at a wavelength of 480nm (blue spectrum) stimulates the cones, rods and ipRGCs29,30 (Figure 7).
Examining the spectral properties of photosensitive pigments in the canine retina, we observe that the use of bright light, at a wavelength of 630nm (red spectrum), stimulates only the rods and cone types containing L/M opsin. The use of bright light at a wavelength of 480nm (blue spectrum) stimulates the cones, rods and ipRGCs29,30 (Figure 7).
The clinical application of these recent discoveries allows the isolation and examination of the outer retinal pathway (cones and rods) separately from the inner retinal pathway (ganglion cells) and optic nerve (Figure 7). This is achieved by a PLR test using red and blue light sequentially,31 termed as a chromatic pupillary light reflex (cPLR) test. In practice, a negative cPLR when evaluated with red light in the 630nm wavelength spectrum, along with a positive cPLR when evaluated with blue light in the 480nm wavelength spectrum, indicates a condition of the outer retinal layer. A negative cPLR in both wavelengths indicates damage in all layers of the retina and/or the optic nerve.12,30,31 The cPLR assessment criteria are the same as those that apply for the assessment of PLR. For the reflex to be considered positive, it should be rapid, complete and continuous.
> The application of the cPLR test in clinical practice
In clinical practice, cPLR is evaluated using light source devices that emit 200 Kcd/m² white, red and blue light beams.12,30 (Figure 8). The test is simple, quick and concerns the assessment of PLR using beams in these three colours sequentially, in a low-light environment. Today, it is usually included in specialised complete ophthalmic examination. cPLR is an extremely reliable reflex that can provide small animal clinical ophthalmologists with valuable information. It facilitates the clinical differential diagnosis of sudden acquired retinal degeneration, progressive retinal degeneration, immunemediated retinopathies, chorioretinopathies, retinal detachment, glaucoma, optic neuritis, meningitis, tumours of the optic chiasm and pituitary gland and visual cortex disorders.12,29,30
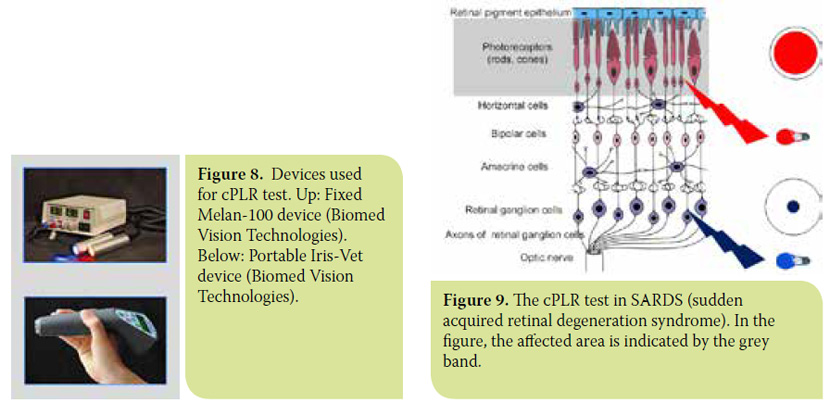
- In sudden acquired retinal degeneration syndrome (SARDS), where necrosis of cones and rods is sudden and complete, the iris remains fully dilated under red light while it contracts normally under blue light (Figure 9).
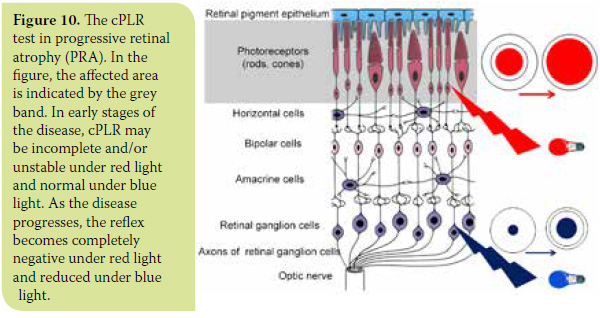
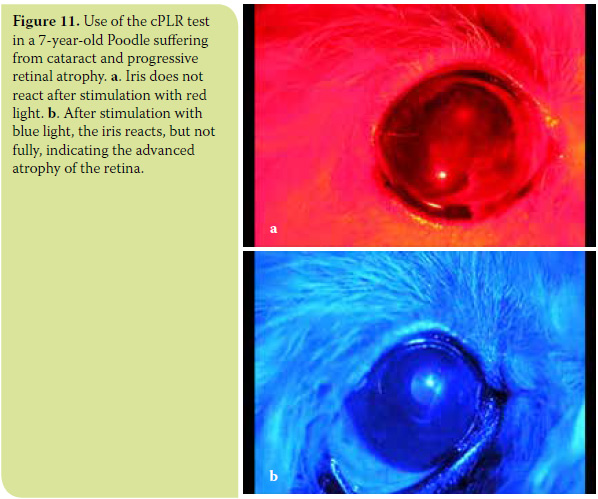
- In the category of diseases described under the general heading Progressive Retinal Atrophy (PRA), where necrosis of cones and rods is progressive, cPLR may be reduced or absent under red light and normal under blue light in the early stages of the disease. With progression of the disease, disturbances are also presented upon stimulation with blue light, so the reflex may be delayed and incomplete (Figure 10). A most common clinical problem in canine ophthalmology concerns the diagnosis of progressive retinal atrophy in animals suffering from cataract, which impedes fundoscopy and evaluation of the retinal condition. The diagnosis of progressive retinal atrophy in these animals is critical in order to avoid unnecessary surgery. Up until now, the preoperative control of retinal function required electroretinography. Nowadays, cPLR, which is unaffected by the presence of cataract, is considered a fast, reliable and inexpensive diagnostic test that does not require general anaesthesia.12,30 (Figure 11). Thus, in some cases, it can complement or replace electroretinography. In patients with a clearly positive cPLR test, electroretinography is not necessary. In those whose cPLR test is not clearly positive, fundoscopy is impossible and/or concomitant ocular disease is suspected, preoperative testing should include electroretinography and ocular ultrasonography.
- In immune-mediated retinopathies, where the proportion of cones and rods affected varies by case, cPLR is often incomplete and/or unstable under red light and normal under blue light.
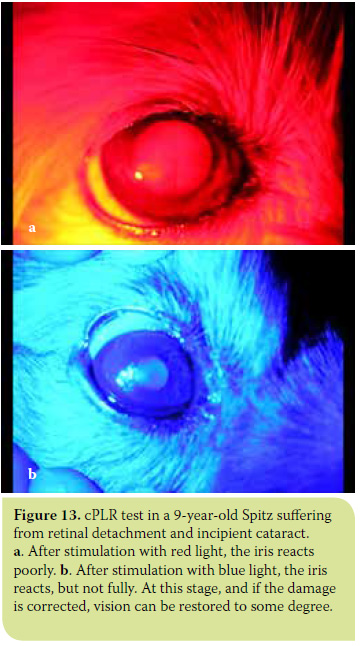
- In chorioretinopathies due to various causes, and in retinal detachment where both the outer (cones and rods) and the inner (ganglion cells) retinal layers are affected. In these cases, cPLR is incomplete and/or unstable under both colours, with slightly better results under blue light due to the increased sensitivity of cones and rods compared to ganglion cells. In the initial stages of these diseases, cPLR may be normal under blue light.30 (Figures 12 and 13).
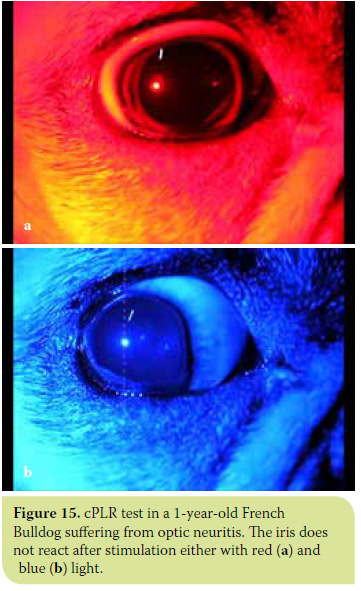
- In all diseases involving the optic nerve and/ or optic chiasm (glaucoma, optic neuritis, meningitis and tumours of the optic chiasm and pituitary gland), cPLR is negative under both red and blue light.30 (Figures 14 and 15).
- Finally, in diseases of the visual cortex, cPLR is positive under both red and blue light stimulation.
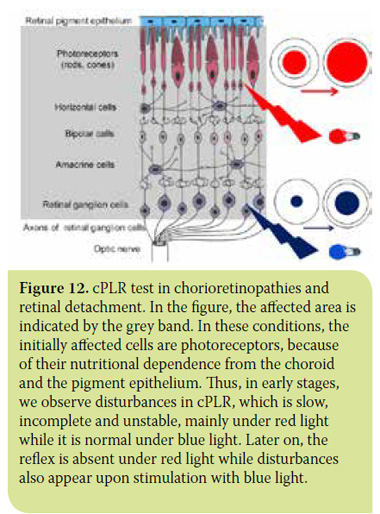
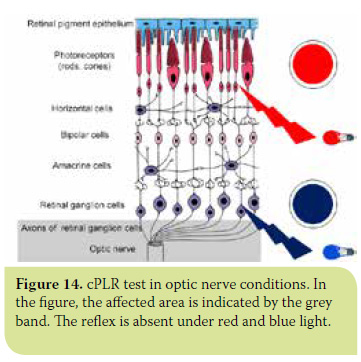
> Conclusion
cPLR is a new diagnostic technique in small animal ophthalmology, which is particularly effective in the differentiation and diagnosis of retinal and optic nerve diseases. Both the literature and the author’s experience in the use of the cPLR test over the last four years have shown it to be a simple and fast method that can be performed in many different settings. Although the test requires the use of special equipment, its cost is not prohibitive. Compared to electroretinography, the cPLR test does not require sedation or general anaesthesia, nor does it involve prolonged dark adaptation, thus enabling its application during routine ophthalmological examination with minimum stress to the animals.12 The cPLR disadvantages are the same as those mentioned for PLR and relate primarily to the presence of diseases of the iris and/ or use of medicines affecting their reliability.
> References
1. Samuelson DA. Ophthalmic anatomy. In: Veterinary Orhthalmology, Gelatt KN (ed). 5th edn. Wiley-Blackwell Publishing: Ames Iowa, 2013, pp. 39-170.
2. Ofri R. Optics and physiology of vision. In Veterinary Orhthalmology, Gelatt KN (ed). 5th edn.Wiley-Blackwell Publishing: Ames Iowa, 2013, pp. 208-270.
3. Jacobs GH. The distribution and nature of colour vision among the mammals. Biol Rev Camb Philos Soc. 1993, 68(3): 413-471.
4. Jacobs GH, Deegan JF 2nd, Crognale MA, Fenwick JA. Photopigments of dogs and foxes and their implications for canid vision. Visual Neuroscience 1993, 10: 173-180.
5. Neitz J, Geist T, Jacobs GH. Color vision in the dog. Visual Neuroscience 1989, 3(2): 119-125.
6. Goodhill GJ, Xu J. The development of retinotectal maps:a review of models based on molecular gradiens. Network 2005, 16(1):5-34.
7. Dean E. Techniques d’examen de l’oeil. In Ohptalmologie du chien, PMCAC ed. Supplement No4, 1997: 21-38.
8. Webb AA, Cullen CL. Neuro-ophthalmology. In Veterinary Orhthalmology, Gelatt KN (ed). 5th edn. Wiley-Blackwell Publishing: Ames Iowa, 2013, pp. 1820-1896.
9. Scagliotti RH, Comparative neuro-ophthalmology. In Veterinary Orhthalmology,.Gelatt KN (ed). 3th edn.Lippincott Williams&Wilkins Publishing: Baltimor, 1999, pp. 1307-1400.
10. Kardon R. Pupillary light reflex. Current Opinion in Ophthalmology 1995, 6: 20-26.
11. Wilhelm H. Neuro-ophthalmology of papillary function – practical guidelines. Journal of Neurology 1998, 245: 573-583.
12. Grozdanic SD, Kecova H, Lazic T Rapid diagnosis of retina and optic nerve abdormalities in canine patients with and without cataracts using chromatic pupil light reflex testing. Vet Ophtalmol, 2013, 5: 329-340.
13. Keeler CE. Blind mice. Journal of experimental zoology 1928, 51(4): 495-508.
14. Foster RG, Provencio I, Hudson D, Fiske S, De Grip W, Menaker M. Circadian photoreception in the retinally degenerate mouse (rd/rd). J Comp Physiol 1991, 169(1): 39-50.
15. Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. Journal of Neuroscience 2000, 20(2): 600-605.
16. Lucas RJ,Douglas RH,Foster RG. Characterization of an ocular photopigment capable of driving papillary constriction in mice. Nat Neurosci. 2001, 4(6): 621-626.
17. Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Menanopsin is required for non-image-forming photic responses in blind mice. Science 2003, 301: 525-527.
18. Qiu X, Kumbalasiri T, Carlson M, Wong Y, Krishna V, Provencio I, Berson M. Induction of photosensitivity by heterologous expression of melanopsin. Nature 2005, 433(7027): 745-749.
19. Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsincontaining retinal gaglion cells: architecture, projections and intrinsic photosensitivity. Science 2002, 295(5557): 1065-1070.
20. Hattar S, Lucas RJ, Mrosovsky N, Tompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofman F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 2003, 424: 76-81.
21. Van Gelder RN, Non-visual photoreception: sensing light without sight. Curr Biol. 2007, 17(24): 2122-2128.
22. Berson DM. Strange vision: ganglion cells as circadian photoreceptors. Trends in Neurosciense 2003, 26: 314-320.
23. Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pocorny J, Tau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signalcolour and irradiance and project to the LGN. Nature 2005, 433(7027): 749-754.
24. Wong KY, Dunn FA, Berson DM. Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron 2005, 48(6): 1001-1010.
25. Zaidi FH, Hull JT, Peirson SN, Wulff K, Aeschbach D, Gooley JJ, Brainard GC, Gregory-Evans K, Rizzo JF, Czeisler CA, Foster RG, Moseley MJ, Lockley SW. Short-wavelength light sensitivity of circadian, papillary and visual awareness in humans lacking an outer retina. Curr Biol.2007, 17(24): 2122- 2128.
26. Berson M. Phototransduction in ganglion-cell photoreceptors. European journal of physiology 2007, 454(5): 849-855.
27. Merkwell EL, Feigl B. Zele AJ. Intrinsicaly photosensitive melanopsin retinal ganglion cell contributions to the papillary light reflex and circadian rhythm. Clin Exp Optom 2010, 93(3): 137-149.
28. Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, Figuero MG, Gamlin PD, Lockley SW, O’Hagan JB, Price LL, Provencio I, Skene DJ, Brainard GC. Measuring and using light in the melanopsin age. Trends Neurosci 2014, 37(1): 1-9.
29. Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A. Chromatic pupil responses: preferential activation of the melanopsinmediated versus outer photoreceptor-mediated pupil light reflex. Ophthalmology 2009, 116: 1564-1573.
30. Grozdanic SD, Matic M, Sakaguchi, Kardon RH. Evaluation of retinal status using chromatic pupil light reflex activity in healthy and diseased canine eyes. Invest Ophthalmol Vis Sci 2007: 48(11): 5178-5183.
31. Park JC, Moura AL, Raza AS, Rhee DW, Kardon RH, Hood DC0. Toward a clinical protocol for assessing rod,cone and malanopsin contributions to the human pupil response. Investigate Ophthalmology & Visual Science 2011, 52: 6624-6635.



