> Abstract
Drug toxicities are relatively common in dogs and cats and they can be classified into type Α or predictable which are caused by the pharmacological or the intrinsic toxic effects of the responsible drug and into type Β or non-predictable that are unrelated to the above. The appearance of type A drug toxicities depends on multiple factors that are related to the affected animal, the dosage regimen and the simultaneous administration of other drugs. Clinical manifestations most commonly originate from organ systems where the responsible drug accumulates or those that are characterized by an increased metabolic rate. In contrast, type B drug toxicities commonly affect organs presenting suitable proteins that after coupling with the drug or its metabolites (haptens) form complete antigens or organs that trap circulating immune complexes. Drugs most commonly responsible for toxicities in dogs and cats include aminoglycosides, macrocyclic lactones (avermectins and milbemycins), pyrethroids, non-steroidal anti-inflammatories, phenobarbital and diazepam.
> Introduction
Drug toxicities in dogs and cats following administration or accidental exposure to various agents are fairly common in the clinic setting. In the U.S.A., the most common cause of poisoning during the period 2002-2010 was the exposure to human medications (approximately 25% of cases), whereas toxicities from ectoparaciticidal and other veterinary medications were included into the 10 most common groups.1
The aim of this article is to present updated information on the classification and the mechanisms that are involved in canine and feline drug toxicities, followed by the presentation of the most commonly responsible drugs or groups of drugs. Only drug-specific treatment guidelines are presented without reference to the general treatment measures (initial stabilization, detoxification, supportive care and symptomatic treatment). Furthermore, there is no reference to cutaneous adverse drug reactions that may present as emergencies (angioedema, Stevens-Johnson syndrome and toxic epidermal necrolysis, cutaneous vasculitis, sterile pustular erythroderma, sulfonamide hypersensitivity syndrome, sterile neutrophilic dermatosis).2
> Classification and pathomechanisms of drug toxicities in dogs and cats
Drug toxicities are classified into these that are caused by the pharmacologic or intrinsic toxic effect of the responsible agent and usually depend on its blood and tissue concentrations (type A or predictable) and those that are unrelated to drug concentrations, occur in a small percentage of treated animals and are usually of immunologic aetiology (type B or unpredictable).3,4 The organs affected by type A toxicities vary according to the agent and they are typically organs where it accumulates, is metabolized and eliminated (e.g. liver, kidneys) or organs with an increased metabolic rate (e.g. bone marrow, intestinal mucosa).3 By contrast, type B toxicities commonly affect organs that express the necessary proteins which bind with the drug or its metabolites (haptens) to form complete antigens (e.g. skin) or organs where circulating antibodies are trapped (e.g. renal glomeruli, joints, blood vessels).3
The appearance of type A drug toxicities depends on multiple factors, including: a) the animal species (e.g. the relative absence of glucuronyl transferase in cats results in increased susceptibility to paracetamol toxicity); b) the metabolic idiosyncrasies of the individual dog or cat that may affect the pharmacokinetics of various agents, like the mutation of the gene that synthesizes P-glycoprotein (P-gp) and is responsible for ivermectin toxicity in Collies; c) any pre-existing lesions in the organs that are affected by the toxic drug (“target” organs) and the functional capacity of the organs that are involved in its metabolism and elimination (e.g. liver, kidneys); d) the effect on the latter and also on the “target” organs of the disease condition for which the toxic drug has been is prescribed; e) the active substance and especially its therapeutic index (the difference between the therapeutic and the toxic dose); f) the dosage regimen (dose, frequency, route and duration of administration), like in the case of aminoglycosides where the administration of the same total daily dose divided every 12 or 8 hours will increase their toxic potential; and, g) the simultaneous administration of other drugs that can affect the pharmacokinetics of the toxic substance through interactions on P-gp and cytochrome P450 enzymes or that can increase its concentration in “target” organs.3,4
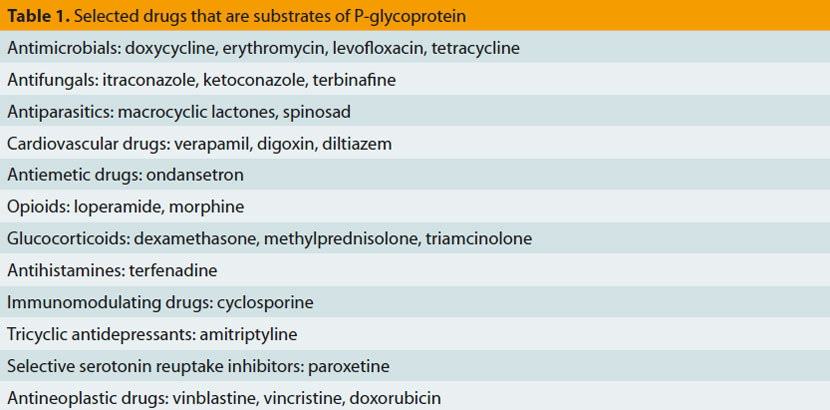
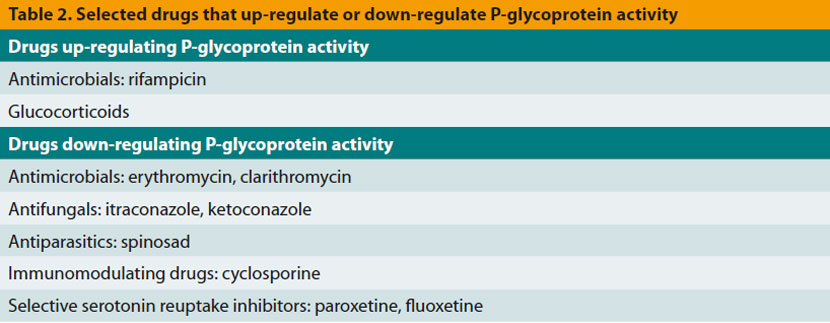
P- glycoprotein is encoded by the ABCB-1 (or MDR1) gene and is extensively expressed on the membrane of multiple cells, including intestinal epithelial cells, capillary endothelial cells of the central nervous system that are part of the bloodbrain barrier, hepatocytes and renal tubular epithelial cells.4-6 Its main biologic role is the reduction of intracellular concentration of various substances, including those drugs that are P-gp substrates (Table 1).4,6,7 Certain drugs can increase the activity of P-gp (Table 2), resulting into reduced intestinal absorption and consequently reduced therapeutic efficacy following oral administration of its substrates.4 Exactly the opposite happens after co-administration of drugs that downregulate the activity of P-gp (Table 2) as well as in the case of ABCB-1 gene mutations, like the nt228 (del4) mutation in dogs of various breeds (Table 3), including Collies.4,6,8-13 In such cases there is an increased potential of toxicity by P-gp substrates, including macrocyclic lactones, loperamide and antineoplastic drugs, due to their increased intestinal absorption, reduced clearance from the central nervous system, and possibly reduced elimination in bile and urine.4,6,14-16 It is emphasized that a dog may be homozygous or heterozygous for nt228 (del4) mutation and that although the possibility of toxicity from the same dose of the drug is higher in the former dogs, it cannot be excluded in the latter; therefore, complete avoidance or dose reduction of P-gp substrates is necessary in both cases.4,8,16 Furthermore, independently of breed of the dog, co-administration of drugs down-regulating P-gp with its substrates must be avoided, especially when the latter have a narrow therapeutic index.3, 6
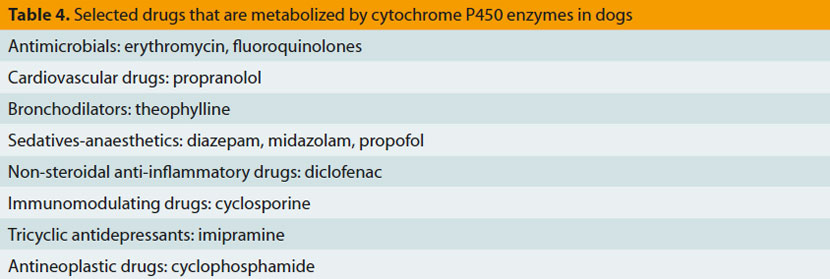
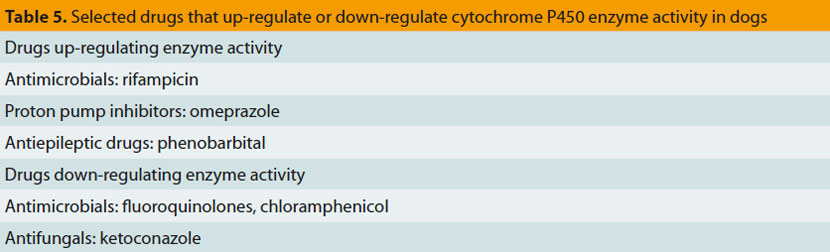
Cytochrome P450 enzymes, responsible for metabolizing hormones, drugs and various chemicals are present in many organs and tissues, including the intestine and the liver.17 In the intestine they metabolize orally administered drugs, thus reducing their bioavailability,4 whereas in liver they catalyse multiple biochemical reactions related to drug metabolism, resulting in the formation of inactive or active metabolites or toxic by-products (Table 4).17,18 The activity of specific cytochrome P450 enzymes that are responsible for metabolism of certain drugs (e.g. enzymes CYP2B11 and CYP2D15) has been found to differ significantly among dogs of the same or of different breeds.4,18,19 Moreover, concomitant administration of drugs that inhibit these enzymes (Table 5) results in reduced metabolism, accumulation and increased toxicity of drugs depending on P-450 cytochrome enzymes for their metabolism.7,17,20-22 Finally, drug interactions on the activity of these enzymes can be used for dose reductions and consequently reduced treatment costs, like in the case of co-administration of cyclosporine with ketoconazole that permits up to 75% reduction of the dosage of the former.7,23,24
> Aminoglycoside toxicity in dogs and cats
Aminoglycosides (e.g. aminosidine, amikacin, gentamicin, kanamycin, neomycin, streptomycin, tobramycin) are effective mostly against Gramnegative aerobic bacteria (e.g. Pseudomonas sp.), but also against some Gram-positives (Staphylococcus sp.) as well as against Leishmania sp. (aminosidine).25 Their bactericidal effect is accomplished following accumulation in the cytoplasm and binding to the 30S ribosome subunit that results in disruption of protein synthesis, increased cell membrane permeability and eventually microorganism death.25 Their bactericidal and postantibiotic effect (PAE) as well as the avoidance of bacterial resistance depend on their maximum concentration and not on the percentage of the dose interval during which their concentrations remain above the minimum inhibitory concentration of the microorganism.25 For this reason, aminoglycosides should be administered in a single daily dose instead of every 8 or 12 hours, as it was practiced in the past.25,26 Until a few years ago, mainly due to their toxicity, aminoglycosides had been largely replaced by fluoroquinolones and were used, almost exclusively, in cases of sepsis.25 However, the use of aminoglycosides has been lately expanded and this trend may continue in the future, for the treatment of canine leishmaniosis (aminosidine) and of methicillin-resistant S. pseudintermedius infections (usually amikacin).27
Aminoglycoside toxicity in dogs and cats is a type A toxicity, targeting mainly the kidneys and inner ear (affecting hearing and balance) and rarely the neuromuscular synapses.3,25 Nephrotoxicity occurs because of their elimination through the kidneys, where they accumulate in tubular epithelial cells resulting into their death, and they inhibit phospholipase activity, necessary for prostaglandine synthesis, resulting in vasoconstriction and reduction of glomerular filtration rate.3,25 The factors which increase nephrotoxic potential are many, including active ingredient (gentamicin is considered more nephrotoxic than amikacin), administration every 8 or 12 hours instead of every 24 hours, prolonged duration of treatment, preexisting kidney lesions, reduced renal blood flow, presence of acidosis and electrolyte imbalances (hypokalaemia, hypernatremia) and simultaneous administration of other nephrotoxic drugs or furosemide.25,27 The most reliable early indicator of kidney damage is increased activity in the urine of those enzymes that are released after necrosis of tubular epithelial cells, like gamma-glutamyl transferase (γ-GT), N-acetyl-b-glucosaminidase (NAG), alanine aminotransferase (ALT) and alkaline phosphatase (ALP),3,25,27,28 whereas more advanced stages present with casts, decreased urine specific gravity, proteinuria, glucosuria and azotemia.3,25,26
Treatment, other than drug discontinuation, is identical to that of acute renal failure except that furosemide is contraindicated in case of oliguria because it will increase the accumulation of aminoglycosides in renal tubular epithelial cells.3 Prevention includes the avoidance of all those factors that increase the likelihood of toxicity and ideally dosage regimen individualization based on active substance concentration in the serum just before the next administration (e.g. it must be lower than 6 μg/ml for amikacin).3,27,29 Unfortunately the latter is not usually feasible in the clinical setting. For this reason, clinical evaluation of the hydration status, urinalysis (with particular attention to protein/creatinine ratio, enzymuria, and microscopic examination of urine sediment for casts) and biochemical assessment of kidney excretory function (blood urea nitrogen, creatinine, and inorganic phosphorus concentration) should be performed in all treated dogs and cats every 7 days during the course of treatment and a week after its completion.3,27 Moreover, co-administration of antioxidants, like silymarin [20mg/ kg body weight (B.W.) daily, per os] and vitamin Ε (25mg/kg B.W. daily, per os) may reduce the renal toxicity of aminoglycosides.30
The ototoxicity, which is usually irreversible and appears more commonly with streptomycin compared to the other drugs of this group, is caused by their prolonged accumulation in perilymph and the consequent metabolic imbalances, including mainly the oxidative damage from free radicals.3,25,31 It may occur after systemic or topical administration, manifesting as deafness or peripheral vestibular syndrome or both which also depends on the specific aminoglycoside (e.g. amikacin and kanamycin have increased toxic potential for the organ of hearing, whereas streptomycin and gentamicin target mainly the balance).25,31 Ototoxicity potential depends on the frequency of administration, treatment duration, and simultaneous use of other ototoxic medication.
Diagnosis is based on acuometry (deafness), an examination not widely used in the clinical setting (however, nowadays there are clinics in our country where it can be performed) and the clinical examination (peripheral vestibular syndrome), whereas the only possible therapeutic measure is immediate treatment discontinuation.25,31 On a preventative basis, simultaneous administration of furosemide is contraindicated because it will increase the concentration of aminoglycosides in the perilymph, and concomitant use of antioxidants may be helpful although, in contrast to nephrotoxicity, this has not been proven for the ototoxic effect of aminoglycosides in dogs and cats.31
Neurotoxicity is rare and occurs because of the inhibition of acetylcholine release in the neuromuscular synapses.3,25 General anaesthesia is a predisposing factor and symptoms are characterized by flaccid paresis or paralysis of all limbs, even progressing in respiratory failure due to respiratory muscle weakness.3,25 Treatment consists of slow intravenous administration of calcium (calcium chloride at 10-20 mg/kg B.W. or calcium glyconate at 30-60 mg/kg B.W.) and perhaps neostigmine (0.04-0.05 mg/kg B.W. subcutaneously or intramuscularly) or edrophonium chloride (0.1- 0.2 mg/kg B.W. intravenously).25
> Macrocyclic lactone toxicity in dogs and cats
Macrocyclic lactones, including avermectins (abamectin, doramectin, eprinomectin, ivermectin, selamectin) and milbemycins (milbemycin, moxidectin, nemadectin) are broad-spectrum anthelminthic parasiticides, effective mainly against nematode worms (including microfilariae) and arachnids and less effective against insects.6 These molecules act mainly as chloride channel agonists but they also increase the release of gamma-aminobutyric acid in sensitive parasites, causing their paralysis and eventually death.6 There are various commercially available formulations (for oral, parenteral, topical administration and as spot-on solution that is systemically absorbed), sometimes in combination with other antiparasitic drugs, with various indications and contraindications, depending on the formulation, suggested dosage regimen and target animal species.6 In an everyday clinic practice they are used very frequently for the prevention and treatment of a large number of parasitic infestations of dogs and cats.
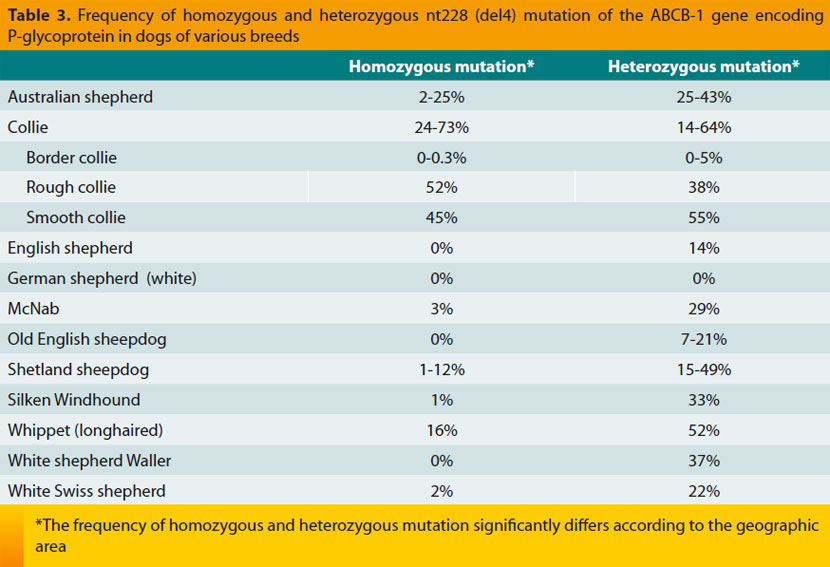
They are safe when administered according to the manufacturer’s instructions.6,32,33 In contrast, use of formulations intended for farm animals and administration at high doses may cause neurotoxicity (type Α toxicity).6,34 For example, ivermectin was the agent responsible for 2.1% of intoxications in companion animals recorded in the U.S.A. during 2009.1 Neurotoxicity occurs due to increased concentration of these substances in the host’s central nervous system, where chloride channels and gamma-aminobutyric acid do exist, and it involves similar mechanisms to those responsible for their paraciticidal effects.6 Symptoms may appear a few hours after the first administration or after several days or even weeks in case of long-standing treatment (e.g. for canine generalized demodicosis) and they are characterized by reduced level of consciousness, disorientation, seizures, mydriasis (or sometimes miosis in cats), blindness (due to retinal oedema), salivation, ataxia, muscle tremors, bradycardia, and even respiratory failure leading to death.3,6,35-43 Macrocyclic lactone neurotoxic potential depends on: a) the active substance, since for example ivermectin and moxidectin are considered to be more toxic than selamectin;32,33 b) dosage regimen and in particular the dose and the duration of administration;38,43 c) presence of ABCB-1 gene mutations decreasing the activity of P-gp.6,34,38,44 For example, Collies homozygous for the nt228 (del4) mutation present signs of toxicity after a single ivermectin administration at the dose of 0.1-0.12mg/kg B.W., heterozygous dogs after prolonged administration at a daily dose of 0.3mg/kg B.W. and dogs without the mutation do not develop toxicity after prolonged use at a daily dose of 0.6mg/kg B.W. or after a single administration at a dose up to 2mg/kg B.W.4,6,42 Similarly, dogs with the same gene mutation present symptoms of toxicity after a single administration of moxidectin at 0.09mg/kg B.W., whereas toxicity appears in dogs without the mutation at dose levels of 1.9-2.8mg/kg B.W.6 On the other hand, ivermectin-sensitive Collies typically do not manifest symptoms of toxicity after milbemycin administration at a dose less than 5mg/kg B.W. and daily doses of 1-2mg/kg B.W. are considered safe, although sporadic cases of toxicity have been reported even with this dosage regimen;6,34 d) concomitant administration of other drugs that inhibit P-gp (Table 2);6,43 and, e) perhaps the age, because very young or very old animals may be more susceptible.6,40 Macrocyclic lactone toxicity is reported more commonly in dogs compared to cats [possibly due to the nt228 (del4) mutation and the more frequent administration at high doses in dogs for the treatment of ectoparasitic skin diseases and especially of generalized demodicosis] and more commonly for ivermectin compared to milbemycin and moxidectin (probably because of the more frequent use of the former at high doses).38,43
Diagnosis is based on history, clinical signs and improvement after drug discontinuation. However, the latter should not be taken for granted and may be seen after several days or even weeks, especially after administration at high doses in dogs with the nt228 (del4) mutation, due to the long elimination half-life of these compounds.6,36,42 Besides symptomatic treatment (e.g. detoxification, antiepileptic medication, mechanical ventilation), 37,42 in severe cases of macrocyclic lactone toxicity in dogs intravenous administration of a 20% lipid emulsion (e.g. Intralipid®) has been employed with encouraging results; the initial dose of 1.5ml/kg B.W. over 1-15min is followed by constant rate infusion at 0.25-0.5ml/kg B.W./ min over 30-60min and the latter can be repeated, once or twice, every 4-6 hours, provided that gross lipemia is not detected in the serum or plasma. 6,35,37,45,46 The most likely mechanism of action of lipid emulsions is binding of these lipid-soluble compounds and enhancement of their elimination from the body.2,6,35,46 Due to lack of sufficient scientific data and of prospective studies and due to the possibility of side effects (hyperlipidemia, hemolysis, lipid embolism, septicemia), this treatment is currently reserved only for those cases with severe symptoms that do not respond to the usual treatment modalities.6,41,45,46 Traditionally, treatment for macrocyclic lactone toxicity includes physostigmine (0.02-0.06mg/kg B.W. or 1 mg/dog, every 12 hours, slowly intravenously) which, despite its short duration of action (30- 90min), allows the animal to regain consciousness and possibly to eat and drink.3,6,47
To avoid macrocyclic lactone toxicity in dogs (independently of their breed) and in cats, only approved formulations and dosage regimens of these drugs should be used. When this is not feasible (for example in dogs with generalized demodicosis when the owner cannot afford the cost of milbemycin treatment), the following measures are recommended: a) toxic doses should not be administered without previous genetic testing for the nt228 (del4) mutation, at least in dogs of high-risk breeds (Table 3); b) the dose should progressively increase (for example in dogs with generalized demodicosis daily oral treatment with ivermectin can be started at 0.1mg/kg B.W. and progressive increase by 0.1mg/kg B.W. up to final dose of 0.6mg/kg B.W.); c) continuous monitoring of the animal during the whole treatment period and immediate discontinuation of the drug if early signs of toxicity, usually mydriasis and salivation, are witnessed; and d) avoidance of concurrent administration of P-gp inhibitors (Table 2).43 It is emphasized that these measures may reduce but they do not eliminate the possibility of toxicity and they may permit timely diagnosis of the latter, which can improve the prognosis.34,38,43,44
> Pyrethroid toxicity in cats
Pyrethroids (e.g. deltamethrin, permethrin) are commonly used in dogs, mostly because their fast and broad spectrum activity against ticks, acari and insects is combined with their insect-repellent properties.48-50 They act at the central nervous system of susceptible parasites and particularly in sodium pumps causing either constant stimulation and spastic paralysis (type 1 pyrethroids like permethrin) or flaccid paralysis after blocking neurotransmission (type 2 pyrethroids like deltamethrin). 48,49 They are commercially available in various formulations (shampoos, spot-on, sprays, collars) mostly approved for dogs only, and they are generally safe in this species when used according to the manufacturer’s instructions.48
Pyrethroid toxicity is fairly common in cats.48,50 Indeed, permethrin toxicity was the most common companion animal poisoning caused by veterinary ectoparaciticidal drugs in the USA during 2009.1 The increased sensitivity of cats is due to reduced activity of the enzymes responsible for pyrethroid metabolism in the liver (type A toxicity) and particularly of glucuronyl transferase.1,49 Toxicity may be observed not only when canine formulations of these compounds are accidentally used in cats, but also when the latter are exposed to recently treated dogs48,49,51-55 and the owners of dogs living along with cats must be made aware of this. Symptoms, usually develop within a few hours or up to three days post-exposure, they are characterized by salivation, generalized muscle tremors, ataxia, hypersensitivity, paraesthesia, excitation and seizures and may result in the animal’s death.48-50,52,55,56 Due to the intense muscle activity, hyperthermia is common whereas myoglobulinuria, which may cause acute renal failure, is less frequently wittnessed.49,55 In case of a positive outcome, recovery is expected after 2-3 days although occasionally it may take up to one week.49,56
Diagnosis is based on history and compatible clinical signs and, at least in theory, may be confirmed by measuring the concentrations of the drug in various tissue samples, including skin and hair.49,50 Treatment includes detoxification (use of a dish washer is recommended for the removal of these lipophilic substances from the skin),48 supportive measures, control of seizures (diazepam, barbiturates, propofol, inhalant anaesthetics)45,48,49,52 and control of muscle tremors with the muscle relaxant methocarbamol (44-50mg/kg B.W., intravenously that may repeated as needed up to a total daily dose of 330mg/kg B.W.).45,48,49,55 Moreover, as in the case of macrocyclic lactone toxicity, intravenous administration of 20% lipid emulsion has been proposed, unfortunately without sufficient scientific evidence to support its effectiveness and safety.46,48,52,57 Prognosis is guarded although approximately 85-95% of cats fully recover, provided that the recommended treatment is instituted in a timely fashion.49,50,52,54,56 Prevention is based on the avoidance of direct or indirect exposure of cats to these substances, especially when at large concentrations, like the spot-on solutions intended to be used on dogs.54,56 For this reason, the author does not recommend the use of the latter in dogs that live in close contact with cats.54
> Non-steroidal anti-inflammatory drug toxicity in dogs and cats
Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used in the clinical setting, mostly for the control of fever, inflammation and pain.58,59 Their main mode of action includes the selective inhibition of cyclooxygenase-2 (COX-2), the necessary enzyme for arachidonic acid metabolism and production of inflammatory mediators like prostaglandins.58,59 Many formulations for veterinary and human use that can be administered topically, parenterally, and orally are commercially available. Although human formulations are more toxic, they are still being used in companion animals, although not as frequently as in the past.
NSAID toxicity occurs mostly due to cyclooxygenase- 1 (COX-1) inhibition, affects mainly the gastrointestinal tract (gastro-duodenal ulcers) and, typically at higher doses, the kidneys (type A toxicity) and it is particularly common.1,58 For example, ibuprofen (Advil®, Algofren®, Brufen®, Nurofen®) was the second most common cause of drug toxicity in dogs and cats in the USA during 2009.1 Gastrointestinal ulceration appears secondarily to the inhibition of synthesis of those prostaglandins that are necessary to control gastric acid secretion, mucus and bicarbonate production and to preserve normal blood flow in the gastrointestinal tract58,59 and is more common when corticosteroids are simultaneously administered. 59 The nephrotoxic effects of NSAIDs are due to inhibition of prostaglandin Ε2 and Ι2 synthesis, which are necessary for vasodilation of renal arterioles, 58,59 and appears more frequently in older animals, when renal blood flow is reduced (like in animals with heart and renal failure and with hypotension) and when other nephrotoxic drugs are administered at the same time.3,58 Moreover, both gastrointestinal ulcers and NSAID nephrotoxicity are more commonly witnessed after administration of human formulation (they contain high drug concentrations and possibly they are less selective inhibitors of COX-2; for example in dogs the toxic dose of ibuprofen is lower than the therapeutic dose),3,58,59 with oral formulations, with higher doses and prolonged duration of administration60 and in cats compared to dogs.58 Finally, it is emphasized that, even with the newer NSAIDs that are approved for companion animals and largely safe, the possibility of toxicity cannot be fully excluded, particularly when some of the above predisposing factors do exist.59
Diagnosis of NSAID toxicity is based on history of administration and clinical signs. Besides detoxification and supportive measures, treatment is that of gastrointestinal ulcers (sucralfate, Η2-blockers or proton pump inhibitors, antiemetics, blood transfusion)3,58 and of acute renal failure (intravenous crystalloids, diuretics, dopamine),58 with the addition of misoprostol (0.002-0.005mg/kg B.W. every 8 to 12 hours, per os), a synthetic analogue of prostaglandin Ε1 that is effective for the treatment of gastrointestinal ulcers and potentially of NSAID-induced nephrotoxicity.3,58,60 Prevention measures include avoidance or exclusion of all predisposing factors and, particularly for high-risk animals, co-administration of misoprostol.3,60
A particular case of NSAID toxicity is carprofen-induced liver toxicity in dogs. This is a type B toxicity that can lead even to liver failure by pathomechanisms that have not been fully elucidated. 3,59,61 Common clinical signs are anorexia, vomiting and jaundice and the main laboratory findings include increased ALP and ALT activities and hyperbilirubinemia.61 Prognosis is relatively good since most dogs show clinical and laboratory improvement following timely discontinuation of the medication coupled with symptomatic treatment and supportive care.61 For this reason, frequent clinical examination and laboratory evaluation of the liver function is recommended, especially when this drug is administered on a long-term basis (e.g. in dogs with osteoarthritis).
Another particular case of NSAID toxicity is due to paracetamol (acetaminophen) in cats and dogs. Paracetamol (Apotel®, Depon®, Panadol®, Protalgon® etc) is broadly used as an analgesic and antipyretic drug in humans that metabolize it through glucuronidation into non-toxic molecules. Paracetamol toxicity in cats was the most common toxicity from human drugs in the USA during 2009.1 It occurs because of the relative lack of glucuronyl transferase activity in this animal species, resulting in paracetamol metabolism initially through the sulfation pathway and then through hydroxylation that produces N-acetylpara- benzoquinoneimine (NAPQUI); the latter is responsible for acute hepatic necrosis, methemoglobulinemia and hemolytic anemia with Heinz body formation.3,62,63 Paracetamol toxicity with similar symptoms has been reported in dogs although less often compared to cats.60,64,65 This is a type A toxicity usually appearing a few hours following administration of a single high dose60 and may be more common in male than female cats.62 Symptoms consist of depression, cyanosis, tachypnea, subcutaneous edema and in severe cases coma and death. Laboratory findings include anaemia, increased ALT and ALP activities and hyperbilirubinaemia.66,67 Treatment includes Nacetylcysteine (intravenous or oral administration at the initial dose of 140-150mg/kg B.W., followed by 50-70mg/kg B.W. every 4-6 hours for 5-17 times); this drug is metabolized into glutathione which binds and removes the toxic metabolites of paracetamol.3,45,60,62,63,66-68 Adjunctive administration of cimetidine (5-10mg/kg B.W. within 48 hours after paracetamol intake) has been proposed to block metabolic enzyme activity and thus to reduce the synthesis of toxic drug metabolites; 3 also liver protectants and antioxidants, like S-adenosyl-L-methionine (SAMe at 20mg/kg B.W. every 24 hours per os) and silymarin (20-50 mg/ kg, every 24 hours per os) may be used.45,65,67 Paracetamol administration, especially in cats, must be avoided at any cost and to this end owner education is highly important, because, most of the time, this human drug is administered without veterinary advice.67
> Phenobarbital toxicity in dogs
Phenobarbital, the first choice drug for the longterm symptomatic treatment of seizures in dogs,69 acts mainly by increasing the activity of gammaaminobutyric acid in the central nervous system. 69 This substance is metabolized in the liver and relatively rarely, following long-term use, may cause hepatotoxicity (type A and possibly type B) that can become lethal, or superficial necrolytic dermatitis (hepatocutaneous syndrome or metabolic epidermal necrosis).3,69-71
The usual symptoms of liver toxicity are depression, anorexia, ascites, and bleeding tendency.70 Diagnosis should not be based only on increased ALP and ALT activities, because this is also witnessed in animals without liver toxicity that are treated with phenobarbital. For this reason, the above laboratory findings should be combined with the increased concentrations of bile acids and possibly of total bilirubin, the decreased concentrations of albumins, blood urea nitrogen and cholesterol and the pathological findings of liver ultrasonography.3,69,70,72,73 Clinical signs and laboratory abnormalities may be reversible if toxicity is diagnosed early and the administration of phenobarbital is discontinued (replacement by another antiepileptic drug) or the dose is reduced, and in every case, by starting symptomatic treatment for liver disease.70 The best preventative measure is regular monitoring, not only of liver function but also of drug concentrations in the blood, starting at 2-3 weeks after the first administration or after any increase in the dosage regimen69 and continuing every 3-6 months, since the potential for toxicity increases when the concentrations are higher than 35-40μg/ml.3,69,70
Superficial necrolytic dermatitis after prolonged phenobarbital administration is characterized by skin lesions, laboratory and diagnostic imaging findings that are indistinguishable from those of the same syndrome not associated with the administration of this drug.71 Diagnosis is based on cutaneous histopathology and liver ultrasonography, treatment is symptomatic and prognosis is guarded.71
> Diazepam toxicity in cats
Diazepam increases the activity of gamma-aminobutyric acid in the central nervous system and has sedative, anxiolytic, antiepileptic and appetite- stimulating properties.69 This molecule can induce an acute and usually lethal liver necrosis (type B toxicity) in the cat by mechanisms that have not been fully elucidated.3,69,74 In fact, diazepam toxicity appears to be more common following short-term treatment as an appetite stimulant (single administration or after a few days), but it is also possible during long-term use (e.g. as an antiepileptic or anxiolytic).3,74,75 Diagnosis is based on clinical signs (lethargy, ataxia, anorexia, jaundice, bleeding tendency) and laboratory evaluation of liver function, treatment is symptomatic and prognosis is guarded to grave.74,75 Measurement of ALP activity before and 5 days post-treatment has been suggested and the drug should be immediately discontinued in case of increased activity in the second sample.74
> Miscellaneous drug toxicities
Many other medications commonly used in companion animal medicine can cause drug toxicities, such as: a) calcium channel blockers (hypotension, cardiac arrhythmias);76 b) angiotensin converting enzyme inhibitors may lead to nephrotoxicity because the lack of angiotensin II results in vasodilation of the efferent renal glomeruli arterioles and thus in reduction of glomerular filtration rate.3 In fact, when prescribed to dogs and cats with heart failure, nephrotoxicity potential increases if this treatment does not lead to a counterbalancing increase of cardiac output;3 c) antineoplastic medications (myelotoxicity, gastrointestinal upset etc.); d) glucocorticoids (gastrointestinal ulcers); e) ketoconazole (hepatotoxicity, especially in cats); 3 f ) loratadine, a second generation antihistamine (reduced or increased level of consciousness, tachycardia);1 and, g) vitamin D, especially human formulations containing cholocalciferol (hypercalcemia, renal failure).
> References
1. McLean MK, Hansen SR. An overview of trends in animal poisoning cases in the United States: 2002-2010. Vet Clin North Am-Small AnimPract 2012, 42: 219-228.
2. Σαριδομιχελάκης Μ. Επείγοντα δερματολογικά περιστα- τικά στο σκύλο και τη γάτα. Ιατρική Ζώων Συντροφιάς 2013, 2: 23-43
3. Boothe DM. Drug-induced diseases. In: Small Animal Clinical Pharmacology and Therapeutics, Boothe DM (ed). 1stedn. WB Saunders: Philadelphia, 2001, pp. 41-59.
4. Mealey KL. Pharmacogenetics.Vet Clin North Am-Small AnimPract 2006, 36: 961-973.
5. Ginn PE. Immunohistochemical detection of P-glycoprotein in formalin-fixed and paraffin-embedded normal and neoplastic canine tissues.Vet Pathol 1996, 33: 533-541.
6. Merola VM, Eubig PA. Toxicology of avermectins and milbemycins (macrocylic lactones) and the role of P-glycoprotein in dogs and cats.Vet Clin North Am-Small AnimPract 2012, 42: 313-333.
7. Palmeiro BS. Cyclosporine in veterinary dermatology.Vet Clin North Am-Small AnimPract 2013, 43: 153-171.
8. Tappin SW, Goodfellow MR, Peters IR, Day MJ, Hall EJ, Mealey KL. Frequency of the mutant MDR1 allele in dogs in the UK.Vet Rec 2012, 171: 72.
9. Neff MW, Robertson KR, Wong AK, Safra N,Broman KW,Slatkin M,Mealey KL,Pedersen NC. Breed distribution and history of canine mdr1-1Delta, a pharmacogenetic mutation that marks the emergence of breeds from the collie lineage. ProcNatlAcadSci U S A 2004, 101: 11725-11730.
10. Mealey KL, Bentjen SA, Waiting DK. Frequency of the mutant MDR1 allele associated with ivermectin sensitivity in a sample population of collies from the northwestern United States. Am J Vet Res 2002, 63: 479-481.
11. Hugnet C, Bentjen SA, Mealey KL. Frequency of the mutant MDR1 allele associated with multidrug sensitivity in a sample of collies from France. J Vet PharmacolTher 2004, 27: 227-229.
12. Geyer J, Döring B, Godoy JR, Leidolf R, Moritz A, Petzinger E. Frequency of the nt230 (del4) MDR1 mutation in Collies and related dog breeds in Germany. J Vet PharmacolTher 2005, 28: 545-551.
13. Gramer I, Leidolf R, Döring B, Klintzsch S, Krämer EM, Yalcin E, Petzinger E, Geyer J. Breed distribution of the nt230(del4) MDR1 mutation in dogs. Vet J 2011, 189: 67-71.
14. McEntee M, Silverman JA, Rassnick K, Zgola M,Chan AO,Tau PT,Page RL. Enhanced bioavailability of oral docetaxel by coadministration of cyclosporin A in dogs and rats.Vet Comp Oncol 2003, 2: 105-112.
15. Sartor LL, Bentjen SA, Trepanier L, Mealey KL. Loperamide toxicity in a collie with the MDR1 mutation associated with ivermectin sensitivity. J Vet Intern Med 2004, 18: 117-118.
16. Mealey KL, Northrup NC, Bentjen SA. Increased toxicity of P-glycoprotein-substrate chemotherapeutic agents in a dog with the MDR1 deletion mutation associated with ivermectin sensitivity. J Am Vet Med Assoc 2003, 223: 1453-1455.
17. Trepanier LA. Cytochrome P450 and its role in veterinary drug interactions.Vet Clin North Am-Small AnimPract 2006, 36: 975-985.
18. Court MH, Hay-Kraus BL, Hill DW, Kind AJ, Greenblatt DJ. Propofol hydroxylation by dog liver microsomes: assay development and dog breed differences. Drug MetabDispos 1999, 27: 1293-1299.
19. Paulson SK, Engel L, Reitz B, Bolten S, Burton EG, Maziasz TJ, Yan B, Schoenhard GL. Evidence for polymorphism in the canine metabolism of the cyclooxygenase 2 inhibitor, celecoxib.Drug MetabDispos 1999, 27: 1133-1142.
20. Shou M, Norcross R, Sandig G, Lu P, Li Y, Lin Y, Mei Q,Rodrigues AD, Rushmore TH. Substrate specificity and kinetic properties of seven heterologously expressed dog cytochromes p450. Drug MetabDispos 2003, 31: 1161-1169.
21. Hirt RA, Teinfalt M, Dederichs D, van den Hoven R. The effect of orally administered marbofloxacin on the pharmacokinetics of theophylline.J Vet Med A 2003,50: 246-250.
22. Lu P, Singh SB, Carr BA, Fang Y, Xiang CD, Rushmore TH,Rodrigues AD,Shou M. Selective inhibition of dog hepatic CYP2B11 and CYP3A12. J PharmacolExpTher 2005, 313: 518-528.
23. Patricelli AJ, Hardie RJ, McAnulty JE. Cyclosporine and ketoconazole for the treatment of perianal fistulas in dogs.J Am Vet Med Assoc 2002, 220: 1009-1016.
24. Gray LL, Hillier A, Cole LK, Rajala-Schultz PJ. The effect of ketoconazole on whole blood and skin ciclosporin concentrations in dogs.Vet Dermatol 2013, 24: 118-125.
25. Dowling PM. Aminoglycosides. In: Antimicrobial Therapy in Veterinary Medicine.Giguere S, Prescott JF, Baggot JD, Walker RD,Dowling PM (eds). 4th edn. Blackwell Publishing: Ames, Iowa, 2006, pp. 207-229.
26. Albarellos G, Montoya L, Ambros L, Kreil V, Hallu R, Rebuelto M. Multiple once-daily dose pharmacokinetics and renal safety of gentamicin in dogs. J Vet PharmacolTher 2004, 27: 21-25.
27. Noli C, Morris D. Guidelines on the use of systemic aminoglycosides in veterinary dermatology. VetDermatol 2011, 22: 379-380.
28. Grauer GF, Greco DS, Behrend EN, Mani I, Fettman MJ, Allen TA. Estimation of quantitative enzymuria in dogs with gentamicin-induced nephrotoxicosis using urine enzyme/ creatinine ratios from spot urine samples.J VetInterMed 1995, 9: 324-327.
29. Frazier DL, Aucoin DP, Riviere JE. Gentamicin pharmacokinetics and nephrotoxicity in naturally acquired and experimentally induced disease in dogs. J Am Vet Med Assoc 1988, 192: 57-63.
30. Varzi HN, Esmailzadeh S, Morovvati H, Avizeh R, Shahriari A, Givi ME. Effect of silymarin and vitamin E on gentamicininduced nephrotoxicity in dogs.J Vet PharmacolTher 2007, 30: 477-481.
31. Oishi N, Talaska AE, Schacht J. Ototoxicity in dogs and cats. Vet Clin North Am-Small AnimPract 2012, 42: 1259-1271.
32. Krautmann MJ, Novotny MJ, De Keulenaer K, Godin CS,Evans EI,McCall JW,Wang C,Rowan TG,Jernigan AD. Safety of selamectin in cats.Vet Parasitol2000, 91: 393-403.
33. Novotny MJ, Krautmann MJ, Ehrhart JC, Godin CS,Evans EI,McCall JW,Sun F,Rowan TG,Jernigan AD. Safety of selamectin in dogs.Vet Parasitol 2000, 91: 377-391.
34. Barbet JL, Snook T, Gay JM, Mealey KL. ABCB1-1 Delta (MDR1-1 Delta) genotype is associated with adverse reactions in dogs treated with milbemycinoxime for generalized demodicosis. Vet Dermatol 2009, 20: 111-114.
35. Clarke DL, Lee JA, Murphy LA, Reineke EL. Use of intravenous lipid emulsion to treat ivermectintoxicosis in a Border Collie. J Am Vet Med Assoc 2011, 239: 1328-1333.
36. Tranquilli WJ, Paul AJ, Todd KS.Assessment of toxicosis induced by high-dose administration of milbemycinoxime in collies.Am J Vet Res 1991, 52: 1170-1172.
37. Crandell DE, Weinberg GL. Moxidectintoxicosis in a puppy successfully treated with intravenous lipids. J Vet EmergCrit Care 2009, 19: 181-186.
38. Merola V, Khan S, Gwaltney-Brant S. Ivermectintoxicosis in dogs: a retrospective study. J Am AnimHospAssoc 2009, 45: 106-111.
39. Kenny PJ, Vernau KM, Puschner B, Maggs DJ. Retinopathy associated with ivermectintoxicosis in two dogs. J Am Vet Med Assoc 2008, 233: 279-284.
40. Lewis DT, Merchant SR, Neer TM. Ivermectintoxicosis in a kitten.J Am Vet Med Assoc 1994, 205: 584-586.
41. Wright HM, Chen AV, Talcott PA, Poppenga RH, Mealey KL. Intravenous fat emulsion as treatment for ivermectintoxicosis in three dogs homozygous for the ABCB1-1Δ gene mutation.J Vet EmergCrit Care 2011, 21: 666-672.
42. Hopper K, Aldrich J, Haskins SC. Ivermectin toxicity in 17 collies. J Vet Intern Med 2002, 16: 89-94.
43. Bissonnette S, Paradis M, Daneau I, Silversides DW. The ABCB1-1Delta mutation is not responsible for subchronic neurotoxicity seen in dogs of non-collie breeds following macrocyclic lactone treatment for generalized demodicosis. Vet Dermatol2009, 20: 60-66.
44. Han JI, Son HW, Park SC, Na KJ. Novel insertion mutation of ABCB1 gene in an ivermectin-sensitive Border Collie. J Vet Sci 2010, 11: 341-344.
45. Khan SA. Common reversal agents/antidotes in small animal poisoning.Vet Clin North Am-Small AnimPract 2012, 42: 403-406.
46. Gwaltney-Brant S, Meadows I. Use of intravenous lipid emulsions for treating certain poisoning cases in small animals. Vet Clin North Am-Small AnimPract 2012, 42: 251-262.
47. Tranquilli WJ, Paul AJ, Seward RL, Todd KS, Dipietro JA. Response to physostigmine administration in collie dogs exhibiting ivermectintoxicosis. J Vet PharmacolTher 1987,10: 96-100.
48. Wismer T, Means C. Toxicology of newer insecticides in small animals. Vet Clin North Am-Small AnimPract 2012, 42: 335- 347.
49. Richardson JA. Permethrin so-on toxicose in cats.J Vet EmergCrit Care 2000, 10: 103-106.
50. Dymond NL, Swift IM. Permethrin toxicity in cats: a retrospective study of 20 cases. Aust Vet J 2008, 86: 219-223.
51. Turner V, Chaffey C, Ferrao P. A survey for small animal veterinarians regarding flea and tick control pesticide products. Can Vet J 2011, 52: 1080-1082.
52. Brückner M, Schwedes CS. Successful treatment of permethrintoxicosis in two cats with an intravenous lipid administration. TierärztlPraxAusg K Kleintiere Heimtiere 2012, 40: 129-134.
53. Linnett PJ. Permethrin toxicosis in cats. Aust Vet J 2008, 86: 32-35.
54. Malik R, Ward MP, Seavers A, Fawcett A, Bell E, Govendir M, Page S.Permethrin spot-on intoxication of cats: literature review and survey of veterinary practitioners in Australia. J Feline Med Surg 2010, 12: 5-14.
55. Boland LA, Angles JM. Feline permethrin toxicity: retrospective study of 42 cases. J Feline Med Surg 2010, 12: 61- 71.
56. Sutton NM, Bates N, Campbell A. Clinical effects and outcome of feline permethrin spot-on poisonings reported to the Veterinary Poisons Information Service (VPIS), London. J Feline Med Surg 2007, 9: 335-339.
57. Haworth MD, Smart L. Use of intravenous lipid therapy in three cases of feline permethrintoxicosis. J Vet EmergCrit Care 2012, 22: 697-702.
58. Khan SA, McLean MK. Toxicology of frequently encountered nonsteroidal anti-inflammatory drugs in dogs and cats. Vet Clin North Am-Small AnimPract2012,42: 289-306.
59. Clark TP. The clinical pharmacology of cyclooxygenase-2- selective and dual inhibitors.Vet Clin North Am-Small AnimPract 2006, 36: 1061-1085.
60. Villar D, Buck WB, Gonzalez JM. Ibuprofen, aspirin and acetaminophen toxicosis and treatment in dogs and cats.Vet Hum Toxicol1998, 40: 156-162.
61. MacPhail CM, Lappin MR, Meyer DJ, Smith SG, Webster CR, Armstrong PJ. Hepatocellular toxicosis associated with administration of carprofen in 21 dogs. J Am Vet Med Assoc 1998,212: 1895-1901.
62. Rumbeiha WK, Lin YS, Oehme FW. Comparison of N-acetylcysteine and methylene blue, alone or in combination, for treatment of acetaminophen toxicosis in cats.Am J Vet Res 1995, 56: 529-533.
63. Gaunt SD, Baker DC, Green RA. Clinicopathologic evaluation N-acetylcysteine therapy in acetaminophen toxicosis in the cat. Am J Vet Res1981, 42: 1982-1984.
64. MacNaughton SM. Acetaminophen toxicosis in a Dalmatian. Can Vet J 2003,44: 142-144.
65. Wallace KP, Center SA, Hickford FH, Warner KL, Smith S. S-adenosyl-L-methionine (SAMe) for the treatment of acetaminophen toxicity in a dog. J Am AnimHospAssoc 2002, 38: 246-254.
66. St Omer VV, McKnight ED. Acetylcysteine for treatment of acetaminophen toxicosis in the cat. J Am Vet Med Assoc 1980, 176: 911-913.
67. Avizeh R, Najafzadeh H, Razijalali M, Shirali S. Evaluation of prophylactic and therapeutic effects of silymarin and N-acetylcysteine in acetaminophen-induced hepatotoxicity in cats. J Vet PharmacolTher 2010, 33: 95-99.
68. Savides MC, Oehme FW, Leipold HW. Effects of various antidotal treatments on acetaminophen toxicosis and biotransformation in cats.Am J Vet Res 1985, 46: 1485-1489.
69. Dewey CW. Anticonvulsant therapy in dogs and cats. Vet Clin North Am-Small AnimPract2006, 36: 1107-1127.
70. Dayrell-Hart B, Steinberg SA, VanWinkle TJ, Farnbach GC. Hepatotoxicity of phenobarbital in dogs: 18 cases (1985-1989). J Am Vet Med Assoc 1991, 199: 1060-1066.
71. March PA, Hillier A, Weisbrode SE, Mattoon JS, Johnson SE, DiBartola SP, Brofman PJ.Superficial necrolytic dermatitis in 11 dogs with a history of phenobarbital administration (1995- 2002).J Vet Intern Med 2004,18: 65-74.
72. Gaskill CL, Miller LM, Mattoon JS, Hoffmann WE, Burton SA, Gelens HC, Ihle SL, Miller JB, Shaw DH, Cribb AE.Liver histopathology and liver and serum alanine aminotransferase and alkaline phosphatase activities in epileptic dogs receiving phenobarbital.Vet Pathol 2005, 42: 147-160.
73. Müller PB, Taboada J, Hosgood G, Partington BP,VanSteenhouse JL,Taylor HW,Wolfsheimer KJ. Effects of longterm phenobarbital treatment on the liver in dogs.J Vet Intern Med 2000, 14: 165-171.
74. Center SA, Elston TH, Rowland PH, Rosen DK, Reitz BL, Brunt JE, Rodan I, House J,Bank S,Lynch LR,Dring LA,Levy JK. Fulminant hepatic failure associated with oral administration of diazepam in 11 cats. J Am Vet Med Assoc 1996,209: 618-625.
75. Park FM. Successful treatment of hepatic failure secondary to diazepam administration in a cat. J Feline Med Surg 2012, 14: 158-160.
76. Hayes CL, Knight M. Calcium channel blocker toxicity in dogs and cats. Vet Clin North Am-Small AnimPract 2012, 42: 263-277.



