Tsitsilianou Al. DVM, Companion Animal Clinic, School of Veterinary Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece | Kazakos G. DVM, PhD, Αssociate Professor of Anesthesia and Intensive Unit Care, Companion Animal Clinic, School of Veterinary Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece | Kotsidou M. DVM, Companion Animal Clinic, School of Veterinary Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
MeSH keywords: anaesthesia, analgesia, epidural, opioids, local anaesthetics
Abstract
Epidural anaesthesia - analgesia (EAA) is a frequently practised regional anaesthetic technique in both human and small animal anaesthesia. Epidural anaesthesia refers to the injection of local anaesthetics in the epidural space for perioperative desensitization of a surgical site producing sensory, motor and autonomic block. Epidural analgesia refers to the injection of opioids in the epidural space for perioperative and postoperative pain management. Its use in small animal clinical practice provides optimum conditions for a plethora of soft tissue and orthopaedic surgeries. EAA may provide a better quality of anaesthesia by decreasing pain and thus anaesthetic and analgesic requirements. Depending on the drug selection EAA can contribute to perioperative stability in anaesthesia, as well as postoperative patient comfort. EAA in dogs and cats is performed in various locations depending on the desired dermatome which needs to be anaesthetized. This can be cervical, thoracic, lumbar, sacro-coccygeal and coccygeal. The most common site for an epidural injection is between the seventh lumbosacral vertebra and the first sacral vertebra. Epidural administration of local anaesthetics and opioids can be implemented by a series of different techniques including the hanging drop technique, the loss of resistance, the detection of extradural pressure waves and more. A minimum volume of 0,2mlkg-1 is required to achieve the cranial spread of the local anaesthetic and the desired epidural blockade. This volume is usually measured based on body weight, but there is also another technique depending on the occipito – coccygeal distance. The commonly administered local anaesthetics include lidocaine, bupivacaine, ropivacaine. Morphine, buprenorphine, methadone, and tramadol, have been used as well. The epidural blockade might induce some side effects depending on the drug selection, including hypoventilation secondary to respiratory depression, cardiovascular depression, neurological complications, opioid-related pruritus and urinary retention. Some important possible complications include technical failure, contamination and hematoma formation. This review aims to provide an update on the EAA technique, the commonly administered local anaesthetics and their combinations, and the possible adverse effects which should be taken into consideration. While initially challenging, is a minimally invasive technique which can be included in a balanced anaesthetic protocol with a perioperative pain management plan.
Ιntroduction
Epidural injection of anaesthetic and analgesic drugs is a frequently used regional anaesthetic technique in small animal practice providing anaesthesia and analgesia to a number of surgical procedures. Lumbosacral epidural anaesthesia and analgesia provides anaesthesia and analgesia caudal to the umbilicus in dogs and cats (Garcia – Pereira 2018). Epidural anaesthesia provides preemptive analgesia by suppressing central sensitization, decreasing pain and inhalant and/or opioid requirements perioperatively (Steagall et al. 2017). Furthermore, it reduces the need for postoperative rescue analgesia, as well as the plasma concentrations of stress - related biomarkers, glucose and cortisol, during surgery (Romano et al. 2016, Steagall et al. 2017). Simple, safe, and inexpensive, this technique remains a useful tool for the clinician with a rather low rate of complications (Jones 2001).
This review is separated in the following sections:
- Anatomy of the epidural space
- Pharmacologic effects of local anaesthetics
- Volume and distribution within the epidural space
- Cardiovascular effects
- Metabolism and hormones
- Technique
- Animal Positioning
- Anatomic landmarks for lumbosacral epidural anaesthesia
- Indications and Contraindications
- Methods to confirm epidural needle placement
- Basic equipment for epidural anaesthesia
- Injected Volumes based on body weight & spinal length
- Body weight
- Spinal length
- Commonly administered drugs and drug combinations – Local anaesthetics
- Opioids
- Adverse Reactions
Anatomy of the epidural space (Figure 1)
The vertebral canal encompasses the epidural space, the meninges (the dura mater, the arachnoid, and the pia mater), the cerebrospinal fluid and the spinal cord. The spinal cord passes through the vertebral canal from the brain to the caudal lumbar region tapering into the conus medullaris (Jones 2001). Distal to this end of the spinal cord is the cauda equina. The dura mater continues caudally past the termination of the spinal cord and forms a membranous sheath referred to as the dural sac. The dural sac typically ends at the level of 6th Epidural anaesthesia and analgesia lumbar vertebra (L6) and the 7th lumbar vertebra (L7) in most dogs, and between L7 and 3th sacral vertebra (S3) in most cats (Campoy 2013).
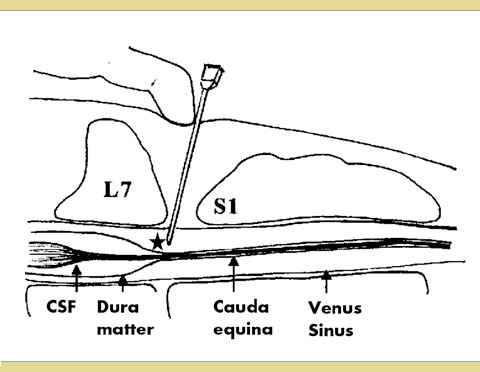
Figure1: Schematic diagram of the epidural space
Star: The tip of the needle is shown in the epidural space.
The epidural space is the “potential” cavity that lies between the dura mater and the surrounding vertebrae. It includes the internal vertebral venous plexus as well as connective and adipose tissues. The epidural space widens at the level of the lumbosacral intervertebral space providing the ideal location for the performance of epidural anaesthesia. There are many sites for epidural anaesthesia, including cervical, thoracic, lumbar, sacrococcygeal and coccygeal, but this review focuses mainly on lumbosacral epidural anaesthesia. Lumbosacral epidural injection of drugs in dogs and cats is performed between L7 and the 1st sacral vertebra (S1) (Jones 2001).
The sacrococcygeal intervertebral space isused for epidural delivery of anaesthetic solutions in cats for desensitization of the perianal area given the fact that the dural sac in cats extends caudally as far as the 1st sacral segment, the sacrococcygeal intervertebral space is a beneficial location where the epidural anaesthesia of the desired area can be done safely and the inadvertent access to the subarachnoid space is prevented. The sacrococcygeal epidural approach eliminates the potential risk for cord puncturing or unintentional intrathecal injection (Garcia – Pereira, 2018).
Pharmacologic effects of local anaesthetics.
Epidural administration of local anaesthetics produces sensory, motor and autonomic blockade. After epidural administration, the anaesthetic solution diffuses across the dura mater and through the intervertebral foramina to act on nerve roots and, finally, block multiple paravertebral nerves. Sensory blockade prevents the nociception, while motor blockade induces profound skeletal muscle relaxation leading to loss of motor function (Campoy 2004, Steagall et al. 2017).
The effect of local anaesthetics on neuronal tissue is related to the size of the nerve fibers and their myelination, the drug concentration achieved and the duration of contact. Small sized, unmyelinated A-δ and C nerve fibers, which are liable for pain transmission, are blocked more efficiently in comparison to myelinated A-α and A-β fibers, which are liable for proprioception, pressure sensation and motor activity. According to this, sensory blockade with minimum impairment of motor function can be achieved with the injection of low concentration of local anaesthetic solutions (Campoy 2004, Steagall et al. 2017). Nevertheless, this is not always true since it is possible that low concentration of a local anaesthetic to cause motor block (Freire et al. 2010). Sensory and motor blockade caused by local anaesthetics is influenced by various factors including lipid solubility and vasoactivity of the local anaesthetic itself, the site of injection and the dose used. Lipid solubility is a factor that can contribute to the onset of action, duration and wear off of the epidural anaesthesia. The addition of a-2 adrenoceptor agonists along with local anaesthetics has a slower onset of action and slower regression of motor blockade than the addition of opioids. An/ For example, morphine has a faster onset of action and a faster regression of motor blockade than dexmedetomidine (Kamal and Talaat 2014).
Volume and distribution within the epidural space
The extent of the epidural blockade depends on the cranial distribution of local anaesthetic drugs and is correlated with the injected volume (Freire et al. 2010). A minimum volume is required to achieve epidural blockade and unless that is administered, potential increase of the local anaesthetic concentration has no further effect. There is no difference whether the volume is injected in the lumbosacral or the sacrococcygeal space. However, the speed of injection affects the cranial spread of the local anaesthetic solution and the pressure generated in the epidural space. During the manual delivery of the injectate, large waves are generated and enhance the cranial spread of the anaesthetic solution in the epidural space (Freire et al. 2010, Garcia- Pereira 2018). The epidural injection can be established via the advancement of an epidural catheter and the use of an infusion pump, but the description of this technique extends the limits of our review (Sasauchi et al. 2016).
Cardiovascular Effects
Sympathetic fibers arise from the 1st thoracic segment (T1) to the 4th lumbar segment (L4) of the spinal cord. Epidural anaesthesia causes disruption of nerve transmission within the spinal cord, the spinal nerve roots as well as the dorsal root ganglia. Epidural anaesthesia which is extended from the level of the low thoracic and lumbar region (T5 – L4) induces a local sympathetic blockade with vascular dilation in the pelvic area and hindlimbs. Autonomic effects are clinically significant if the ganglionic sympathetic blockade extends between the 5th thoracic segment (T5) and the 3rd lumbar segment (L3). Sympathetic and motor blockade may be avoided when opioids, like morphine, are administered epidurally for perioperative pain management. The negative cardiovascular effects will include vasodilation in the afflicted dermatomes with consequent hypotension. The sympathetic blockade leads to peripheral venodilation and reduced cardiac venous return causing hypotension and bradyarrhythmias, the so called “reverse” Bainbridge reflex (Crystal & Salem 2012). When blockade extends at the level of 2nd thoracic segment (T2) to 4th thoracic segment (T4), heart rate and cardiac contractility might be reduced by blockage of the cardiac accelerator nerve fibers (Jones 2001, Campoy 2004).
Metabolism and hormones
Surgical trauma induces an inflammation which leads to a neuroendocrine response that activates the renin-angiotensin system and results in increases in adrenocorticotropic hormone, cortisol, epinephrine, norepinephrine and vasopressin blood concentration. This “stress response” can be totally or partially suppressed, with neuraxial blockade (Almeida et al. 2010). The neuraxial blockades might reduce perioperative arrhythmias by suppressing the “stress response” and decreasing the release of epinephrine and norepinephrine (Campoy 2004). The perioperative stress response contributes to postoperative susceptibility to infections. High levels of cortisol and glucose in circulation caused by insulin resistance following surgical stimulation lead to higher risk for wound infection and impaired wound healing. The total suppression of the adrenal and glycaemic responses may also have a beneficial effect on the patient’s immune system and postoperative recovery (Romano et al. 2016).
Epidural Anaesthesia Technique
Animal Positioning
Epidural anaesthesia may be performed in the sedated, or preferably anaesthetized patient in sternal or lateral recumbency. The sternal recumbency is the preferred position because it offers a better visualization of the epidural space and thus, a higher success rate due to the easier identification of the latter (Jones 2001, Martinez -Taboada & Redondo, 2017). In sternal recumbency, the hind limbs are extended cranially with the spine flexed in a kyphotic position that allows the maximum extension and easier identification of the lumbosacral space (Jones 2001). Most of the commercially available solutions, are hypobaric at body temperature. The total dilution of the local anaesthetics increases their hypobaricity unless glucose or hypertonic saline are added. The epidural injection of hypobaric solutions provokes the migration of local anaesthetics to non dependent areas, while the injection of hyperbaric solutions provokes only the migration of local anaesthetics to dependent areas. However, hyperbaric solutions are sometimes related with higher incidence of neurotoxicity (Ganem et al. 1996).
Anatomic landmarks for lumbosacral epidural anaesthesia
(Figure 2) (Figure 3) (Figure 4)
After positioning the patient in either sternal or lateral recumbency depending on the clinician’s preference and experience, the hair should be clipped over a sufficient area to identify the anatomic landmarks for accurate needle insertion. The sternal recumbency allows for a better visualization of the lumbosacral area, and it’s more preferable for the hanging drop technique. The lateral recumbency may be indicated in cases of patient trauma in the pelvic area (pelvic fractures, etc). The site is located by using the caudal dorsal iliac spines of the pelvis and the dorsal spinous processes of the L7 and the corresponding of the sacrum (Campoy 2004). The external iliac crests are palpated with the thumb and the middle finger of one hand. The index finger of the same hand is directed caudally and palpates the spinous process of the L7 (Wetmore & Glowaski 2000). An imaginary line joining the caudal dorsal iliac spines crosses midline at the lumbosacral junction that can be palpated as a depression between them (Valverde 2008). The dominant hand must place the needle accurately on the midline and caudal to the L7 spinous process, perpendicular to the pelvis plane (Jones 2001). Identification of the anatomic landmarks may be difficult in heavily muscled or obese dogs and dogs with rounded hindquarters. In these patients, the imaginary line follows midline between the L6 and the L7 and crosses the imaginary line joining the iliac crests. The dorsal spinous process of the L7 is palpated and the lumbosacral space is determined as a de- pression directly rostral to it (Jones 2001, Wetmore & Glowaski 2000).
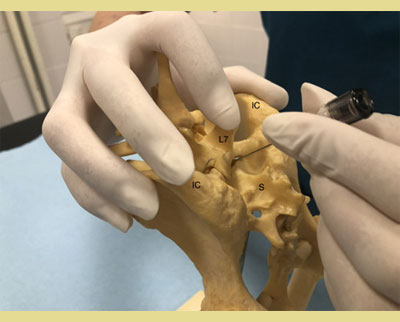
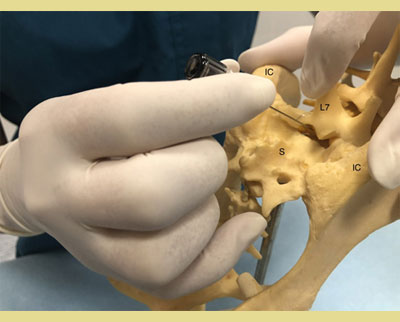
Figure 2. and 3. The external iliac crests are palpated with the thumb and middle finger of one hand. The index finger of the same hand palpates the spinous process of the seventh lumbar (L7) vertebra.
IC: Iliac Crest
L7: 7th Lumbar Vertebra
S: Sacrum
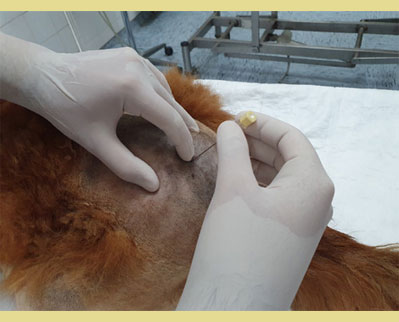
Figure 4. In sternal recumbency, the hind limbs are extended cranially with the spine flexed in a kyphotic position. The external iliac crests are palpated with the thumb and middle finger of one hand. The index finger of the same hand palpates the spinous process of the seventh lumbar (L7) vertebra. The needle is introduced perpendicular to the skin.
In the sacrococcygeal approach particularly, the cat is placed in sternal recumbency with the hindlimbs extended cranially. The sacrococcygeal space can be palpated between the sacrum and the first coccygeal vertebrae. Torruella et al. (2023) stated that the moving of the tail up and down may be beneficial for the correct identification of epidural space. The rest of the technique is the same as mentioned above.
Indications and contraindications
Lumbosacral and sacrococcygeal EAA is considered a safe and effective anaesthetic technique in various occasions, such as orthopedic procedures, peri-anal and peri-vulvar surgeries and other soft tissue surgeries caudal to the umbilicus, like caesarean section (Bartel et al. 2016, Campoy 2004) (Table 1).
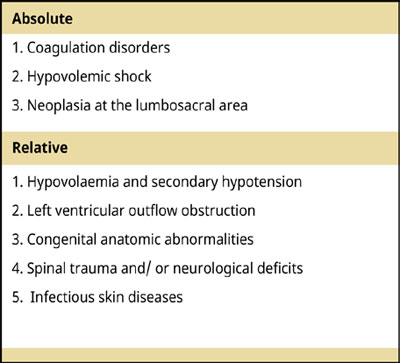
Table 1.
Contraindications.
Epidural anaesthesia has several contraindications. Coagulation disorders is one of those. The epidural space is very well vascularized and an inadvertent needle insertion in a vessel would lead to formation of an epidural or even a subarachnoid hematoma (if the needle penetrates the dura mater) resulting in spinal compression and subsequent neurological deterioration of the hind limbs. Hypovolaemia and profound hypotension are also relative contraindications, and an epidural blockade should not be performed until the patient is haemodynamically stable (Wetmore & Glowaski 2000).
Another major contraindication of epidural anaesthesia is the presence of any type of infectious skin disease, such as pyoderma or dermatitis, at the site of injection. The introduction of pathogenic microorganisms in the epidural space will most likely have a deleterious effect on neural structures. Patients with neoplasia at the lumbosacral area are also excluded from epidural puncture, since the introduction of neoplastic cells would have undesirable consequences, such as scattering of neoplastic cells into the epidural space (Campoy 2004). In addition, although not proven, epidural administration of anaesthetics in patients with severe bacteraemia or sepsis may cause hematogenous transmission of the infection into the epidural or spinal space in case of inadvertent needle or catheter placement (Campoy 2004). However, it is worth mentioning that in human medicine a single epidural injection has been performed in cases of sepsis and it is considered to be beneficial (Tyagi 2017).
Relative contraindications of epidural anaesthesia include left ventricular outflow obstruction, congenital anatomic abnormalities, spinal trauma and neurological deficits (Wetmore & Glowaski 2000, Jones 2001). Patients with valvular aortic stenosis, mitral stenosis or hypertrophic subaortic stenosis lack the ability to raise their cardiac output effectively following hypotension consequent to epidural administration of drugs and this limited response is precarious. Nevertheless, if these patients are medically well controlled and they are not in heart failure, they could benefit from an epidural anaesthesia (Jones 2001, Campoy 2004). Congenital anatomic abnormalities present technical difficulties in epidural anaesthesia due to the potential change in anatomic landmarks that might make needle insertion in epidural space more complicated, like lumbosacral transitional vertebra. In these specific occasions, more advanced techniques for confirmation of the epidural space are used (Jones 2001). Finally, in cases of spinal trauma and/or neurological deficits, epidural injection of drugs is contraindicated in concept of avoiding further complications (Wetmore & Glowaski 2000).
Methods to confirm epidural needle placement
Various methods have been described to confirm correct epidural needle. The two most widely used methods include the “loss of resistance” (LOR) and the hanging drop technique. In “loss of resistance”, the needle is introduced perpendicular to the skin, or at a 30–45° angle in cats, and it is advanced gently until a popping sensation is felt, as the needle pierces the ligamentum flavum. After the removal of the stilette, and if no aspiration of cerebrospinal fluid (CNF) or blood into the syringe is detected, a saline-filled or an air-filled syringe is placed to the hub of the needle, and a small volume (0.25 – 0.50 ml depending on the body weight of the patient) of air or saline is injected into the epidural space. Verification of correct needle placement is positive when injection of air or fluid is performed with no resistance (Valverde 2008, O’Hearn & Wright 2011, Adami & Gendron 2017).
In the “hanging drop” technique, the needle is advanced just close but not in the epidural space and the stilette is removed before the needle pierces the ligamentum flavum. A drop of saline or local anaesthetic is then placed in the hub of the needle and the needle is advanced through the ligamentum flavum until the drop is aspirated into the epidural space due to the subatmospheric pressure that exists in epidural space. This confirms the accurate placement of the needle (Valverde 2008). For some authors the “hanging drop” technique is a quite reliable technique when the patient is positioned in sternal recumbency (Martinez -Taboada & Redondo 2017) (Figure 5, Figure 6).
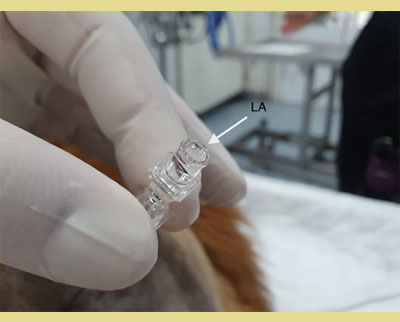
Figure 5. A drop of saline or local anaesthetic is placed in the hub of the needle, after the removal of the stiletto, and the needle is advanced through the ligamentum flavum until the drop is aspirated into the epidural space due to the subatmospheric pressure that exists in epidural space (Hanging Drop Technique). This confirms the accurate placement of the needle.
LA: The Meniscus of Local Anaesthetics
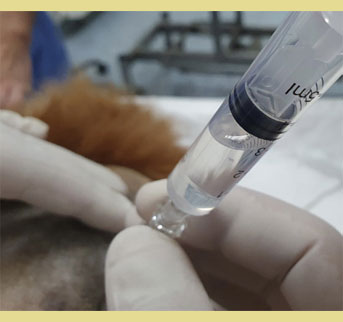
Figure 6. Verification of correct needle placement with the injection of air or fluid with no resistance (Loss of Resistance).
There are more sophisticated methods to verify needle position in epidural anaesthesia. Among those are the use of nerve stimulator, the observation of epidural pressure waves and the use of advanced imaging techniques. It is performed with an electrically insulated spinal needle that is attached to a neurostimulator cathode. Under deep sedation or general anaesthesia, the patient is positioned in either lateral or sternal recumbency with the pelvic limbs extended forward. The anode of the neurostimulator is placed on the patient’s skin over the semitendinosus muscle on the hind limb. The needle is connected to the nerve stimulator and is advanced gently over the lumbosacral area. Electrolocation is based on the principal that the current that is needed to induce motor responses decreases as the needle is introduced into the epidural space. The minimal electric threshold (MET) to generate hind limb muscular contraction and/or tail switches when the epidural space is approached and the epidural lumbosacral plexus is stimulated is 0.3 mA in dogs and 0.7 mA in cats, at a pulse width of 0.1ms. This will confirm the correct needle placement in the epidural space (Otero et al. 2015).
The detection of extradural pressure waves is another promising technique to verify the accurate needle placement into the epidural space. It is performed with a pressure transducer which detects the presence of pressure waves after the correct needle placement in the epidural space (Adami & Gendron 2017). Following the insertion of the spinal needle into the epidural space, it is connected to a pressure transducer via a fluid filled, non-distensible pressure line and the pressure values are recorded. An increase in extradural space pressure and detectable pressure waves is anticipated after the extradural administration of a specific amount of sterile saline, therefore verifying the correct needle placement (Iff & Moens 2010). The epidural pressure waves are like arterial waveforms, so the higher the pressure values the greater the possibility to detect them (Adami & Gendron 2017). A latest update of this technique is a continuous pressure monitor which displays pressure readings and provides information on the thickness and the conformation of the tissue or space where the needle is advanced. By the time the spinal needle is inserted into the epidural space, a sudden drop in needle tip pressure is detected and this can be visualized on display or translated into an acoustic signal (Sasauchi et al. 2016).
The use of ultrasound, contrast CT imaging, radiography and/or fluoroscopy are more sophisticated techniques for verifying the correct needle position in the epidural space. Ultrasound- guided epidural needle and catheter placement in dogs is a feasible method which only requires the acquaintance of the ultrasound anatomic structures of the lumbosacral area in parasagittal and transverse images. It can be performed in normal patients, obese patients and patients with radiographic abnormalities at the lumbosacral space. Contrast medium distribution can also serve as a confirmation method via CT examination or fluoroscopy visualization. Ιn addition, there are two techniques worth to be mentioned for confirmation of the epidural space, the Baraka technique and epidurography. Despite the fact that the gold standard technique is epidurography, for the average clinic the hanging drop technique is an acceptable alternative for epidural anaesthesia and analgesia. (Adami & Gendron 2017).
Basic equipment for epidural anaesthesia (Jones 2001)
- Clippers
- Surgical preparation solutions
- Tuohy needle (22-18-gauge) or spinal needle (20-22-gauge, 2.5-7.5-cm)
- 22-21 gauge spinal needle, 2.5 cm for small dogs and cats (Figure 7)
- 20 gauge spinal needle, 3.8 cm for medium sized dogs
- 18 gauge spinal needle 7.5 cm for large dogs
- Loss of resistance glass syringe
- Sterile isotonic saline solution
- Preservative-free local anaesthetic solution with/ without adjuvant
- Syringes and needles
- Sterile gloves

Figure 7.
Spinal needle 22G a .a
Injected Volumes based on body weight & spinal length
There are two methods which are mainly used to determine the volume of the commonly administered drugs and drug combinations into the epidural space: body weight and spinal length.
Bodyweight
Depending on the desired blockade and the drug choice, the doses for local anaesthetics are adjusted accordingly to block a significant number of spinal nerves. The spread of local anaesthetics is mainly based on the volume injected within the epidural space. It is common the addition of 0.9% saline to reach the volume needed to ensure the rostral spread of epidural anaesthesia and analgesia. The typical final volume for local anaesthetics is usually 0.2 mL kg-1 based on body weight. In example, if a patient weights 20kg, the final volume administered epidurally would be 4 ml based on body weight. This volume injected at the L7-S1 intervertebral space can achieve a blockade up to the thoracolumbar area (Freire et al. 2010). Smaller volumes than 0.2mL kg-1 , which extend to L3, achieve sensory blockade to the pelvis and the pelvic limbs, while larger volumes than 0.2mL kg-1 , which extend to the thoracolumbar area, block sensory innervation to the abdomen. (Valverde & Skelding 2019).
Spinal length
The total vertebral column length (Loc) is calculated from the occipital condyle to the first coccygeal vertebra. When the spinal column length is used to measure the epidural anaesthetic volume, it is calculated as mL cm Loc-1 . A volume of 0.05 mL cm Loc-1 offers a block up to the level of L1. A volume of 0.1 mL cm Loc-1 offers a block up to the level of T9-T10 . A volume of 0.15 mL cm Loc-1 offers a block up to the level of T4 -T5. In example, if a patie’t’s occipito-coccygeal distance is 30 cm, the final volume administered epidurally would be 1.5 ml to reach a cranial block to the level of L1 (Otero et al. 2010).
The calculated volumes based on length of the vertebral column versus body weight tend to be higher in small, medium and large body score condition score (BCS 2.3) dogs, while in large (BCS 4.5) and giant dogs the difference isn’t clinically relevant. Nevertheless, BCS significantly affects the epidural volume of injectate and it should always be taken into consideration (Valverde & Skelding 2019). Since there is not much evidence on the use of spinal length the authors agree that attention should be paid considering that this method is followed by higher volume in small and medium dogs. Further research is needed.
Commonly administered drugs and drug combinations – Local anaesthetics
A number of local anaesthetics of various concentrations, doses, and combinations have been used for the development of epidural anaesthesia, triggering a wide spectrum of sensory and motor blockades. Lidocaine 2% is a frequently used local anaesthetic drug that can be administered epidurally to produce a quick desensitization with good relaxation of the muscles. The typical dose is 5mg/ kg or 2.5 mg kg-1 with the addition of epinephrine (1:200.000), alone and in combination respectively. The onset of time is 4 – 6 minutes and the duration of action lasts 1 hour (Steagall et al. 2017).
Bupivacaine is the most common local analgesic that is used in both dogs and cats to induce long-lasting caudal analgesia (Ferreira et al. 2018). Bupivacaine can be found as a 50:50 racemic mixture of its enantiomers, dextrobupivacaine and levobupivacaine. Levobupivacaine, a synthetic enantiopure solution, has a similar duration of action and a dose-dependent degree of analgesia and motor blockade compared to traditional racemic bupivacaine (Cerasoli et al. 2017). Levobupivacaine also produces fewer arrhythmias and cardiotoxicity than equivalent concentrations of bupivacaine (Groban et al. 2001). There is no agreement among the references regarding bupivacaine doses, concentrations, and volumes (Freire et al. 2010) An epidural injection of 0.25% or 0.75% bupivacaine (0.5 – 1 mg kg-1 ) induces a sensory blockade up to the level of the L3 or L4, respectively (Duke et al. 2000). The onset time is 5 - 15 minutes, and the duration of action is 4 – 12 hours (Grubb & Lobprise 2020, Epidural anaesthesia and analgesia Steagall et al. 2017, Campoy et al 2013).
Ropivacaine is a new generation amino amide local analgesic, morphologically similar to bupivacaine. Despite ropivacaine’s lower liposolubility, it appears to be more effective in blocking A and C fibers (Duke et al. 2000). The less cardiotoxic and arrythmogenic ropivacaine provides an enhanced perioperative analgesia with sufficient blockade of the sensory function, when administered in an epidural mixture with opioids (Groban et al. 2001, Bosmans et al. 2012). Epidural ropivacaine 0.75% alone using 1.65 mg kg-1 can also provide a successful sensory blockade at dermatomes L5 - L7 (Duke et al. 2000), while producing a motor blockade of shorter duration. The onset time is 7–15 minutes, and the duration of action is 5 – 8 hours (Campoy et al. 2013, Steagall et al. 2017, Grubb & Loprsie 2020).
Opioids
The epidural administration of opioids provides worth-mentioning preventive analgesia, while minimizing the risk of adverse effects associated with systemic administration of the same drugs (Troncy et al. 2002). Morphine, a preservative-free opioid with low lipid solubility, is more potent epidurally inducing a higher degree and duration of action (Wetmore & Glowaski 2000, Valverde 2008). The epidural injection of morphine at 0.1 mg kg-1 using 0.1 ml kg-1 could also be combined with local anaesthetics providing better quality analgesia and adequate pain control. The epidural injection of preservative free morphine can be delivered alone in cases where motor and sympathetic block should be avoided. The onset of time is 45 – 90 minutes and the duration of action lasts from 12 to 24 hours (Kona – Boun et al. 2006, Steagall et al. 2017). Even though opioids don’t cause sympathetic or motor blockade, the administration of morphine can still have some side effects including vomiting, mild cardiovascular and respiratory depression, pruritus, delayed hair growth and urinary retention (Troncy et al. 2002).
Oxymorphine hydrochloride is another preservative-free opioid, which is more lipid soluble and 10 times more potent epidurally than morphine. It is administered at 0.1 mg kg-1 in combination with 0.75% bupivacaine 1 mg kg-1 at the final volume of 0.2 ml kg-1 injected at the lumbosacral space. Oxymorphone’s onset time is 20 – 40 minutes and the duration of action lasts from 6 to 10 hours (Steagall et al. 2017). Since oxymorphone induces a central mediated increase in vagal tone, a decrease in heart rate, transient hypotension and contingent respiratory depression are rather anticipated. Oxymorphone may induce a more profound segmen tal effect compared to morphine due to its intermediate lipid solubility and consequent rapid systemic absorption following epidural injection. Therefore, its epidural administration is preferable for surgeries regarding the hindlimb and caudal abdomen Torske et al. 1999). Oxymorphone remains one of the most expensive opioids in veterinary clinical practice. However, there are more economic alternatives to the use of oxymorphone, such as hydromorphone, whose action stands between morphine and oxymorphone (Pettifer & Dyson 2000).
Methadone is another opioid analgesic whose epidural administration induces a more profound and prolonged analgesia than its parenteral administration (Campagnol et al. 2012). Methadone via the epidural route obtunds nociception without motor effects and it may permit decreases in the inhalant anaesthetic requirements for surgical procedures where prolonged nociceptive stimulation takes place (Campagnol et al. 2012). The epidural injection of methadone at the dose of 0–0.5 mg kg-1 has an onset of action of 10 to 20 minutes and its duration of analgesia lasts up to 7 hours (Bosmans et al. 2012). Although cardiopulmonary and sedative effects of intravenously or epidurally administered methadone are similar, the epidural route provides an analgesic effect of longer duration centered on specific dermatomes (Campagnol et al. 2012).
Fentanyl is an opioid frequently employed in human medicine, which can be added in combination with other local anaesthetics, because of its high lipophilicity provides only a short duration of action (Saritas et al. 2014, Steagall et al. 2017).
Butorphanol and buprenorphine are opioids with high lipid solubility which mainly produce a segmental spinal analgesia. Butorphanol at the dose of 0.25 mg kg-1 Epidural anaesthesia and analgesia might be selected for epidural administration due to its lack of cardiovascular side effects. Its onset of time is 10 to 20 minutes and its duration of action lasts from 3 to 4 hours. Buprenorphine at the dose of 4μg kg is a promising alternative to epidural morphine with equal analgesic effect and duration of action (Smith & Kwand 2001, Towers 2020). Its onset of time is less than 45 - 60 minutes and its duration of action lasts up to and greater than 24 hours. The epidural administration of buprenorphine induces long term analgesia with low incident of urinary retention and adequate antinociceptive effect (Steagall et al. 2017, Towers 2020). Based on the concept of multimodal analgesia, buprenorphine may be used in combination with either bupivacaine or α2 adrenergic agents, such as medetomidine, deploying the synergism of local anaesthetics and opioids that provides rapid onset and enhanced analgesia (Steagall et al. 2009, Bartel et al. 2016, Towers 2020).
Alpha-2 adrenergic agonists, such as medetomidine and dexmedetomidine, are well known for their antinociceptive properties and their synergistic interaction with opioids in the spinal cord. Despite the prominent depression of cardiovascular function caused by α2 – adrenergic agonists and the respiratory depression caused by opioids, their epidural combination provides a longer duration of analgesia with fewer side-effects, since lower doses are required to produce the desired blockade (Branson et al. 1993). Medetomidine at the dose of 10μg kg-1 combined with morphine at the dose of 0.11 mg kg-1 can provide analgesia up to 13 hours (Branson et al. 1993). Dexmedetomidine, the active enantiomer of the racemic mixture medetomidine, similarly prolongs the duration and enhances the action of local anaesthetics via a-2A adrenoceptors with minimal effect on motor function (Yoshitomi et al. 2008). The epidural administration of dexmedetomidine at 3 – 6 μg kg-1 contributes to isoflurane MAC reduction in a dose-dependent fashion which may last up to 4.5 hours. Nevertheless, dexmedetomidine may induce bradycardia and/or an increase in arterial blood pressure (Campagnol et al. 2007).
Ketamine is a noncompetitive N-methyl D-aspartate receptor antagonist and reduces spinal cord hyperexcitability and nociception. It is commercially found as a racemic mixture of two enantiomers, S(+) ketamine and R(-) ketamine. S(+) ketamine is the left- handed optical isomer and R(-) ketamine is the right- handed optical isomer. The S(+) ketamine has a 4 times greater affinity for N-methyl D-aspartate receptors than R(-) ketamine. Epidural administration of ketamine in a racemic mixture or S(+) ketamine alone at doses of 1 – 3 mg kg-1 can be used pre-emptively in combination with opioids or other local anaesthetics for treatment of post-incisional hyperalgesia. Even though the S(+) isomer is 2-4 times more potent than the racemic form, the later has a more profound analgesic effect with a prolonged recovery period (Duque et al. 2004). The preventive injection of ketamine can provide motor, sensory and sympathetic blockade with minimal hemodynamic effects and cardiorespiratory depression. Furthermore, ketamine following epidural injection might induce ptyalism, nystagmus, conscious proprioception deficit, ataxia and recumbency of shorter duration than the period of analgesia (DeRossi et al. 2009).
Τramadol is a racemic mixture of two enantiom- ers [(+) and (-)], whose analgesic effect results primarily from the action of M1 enantiomer. The epidural administration of tramadol at the dose of – 1 - 2 mg kg-1 Epidural anaesthesia and analgesia in combination with local anaesthetics produces adequate intra- and post- operative analgesia for 6 to 12 hours (Almeida et al. 2010). Epidural tramadol poses no risk of respiratory depression in comparison to other opioids, such as morphine, which may induce a mild cardiorespiratory depression There is evidence that there is no significant difference between the systemic versus the epidural administration of tramadol (Mastrocinque et al. 2012) (Table 2).
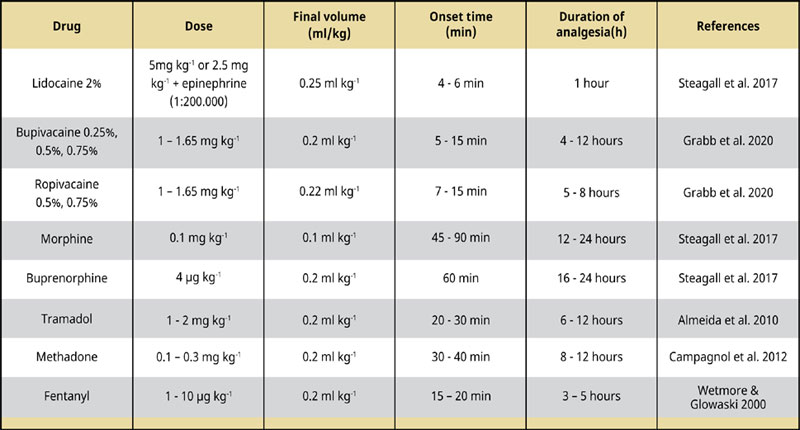
Table 2 Commonly used epidural anaesthetics and analgesics in dogs.
Adverse Effects
Adverse effects associated with epidural anaesthesia are rare when preexisting contraindications are identified in time. Most of the complications rapidly resolve after cessation of the drug effect with minimal additional interventions (Wetmore & Glowaski 2000).
The most common complication is hypoventilation secondary to respiratory depression due to the blockade of the phrenic nerve. The respiratory paralysis has a higher prevalence in obese patients due to the excess of epidural fat and the limited epidural space. The rapid administration rate and the head-down position of the patient are often responsible for the cranial migration of local anaesthetic to more distant neurotomes (Savvas et al. 2016). The inadvertent administration of local anaesthetics into the subarachnoid space may also provoke respiratory depression attributable to the anterior spread of the drug through the cerebrospinal fluid. Thus, the normal epidural doses of local anaesthetics should be halved if the needle is placed into the subarachnoid space (Jones 2001). If respiratory depression due to local anaesthetics or epidural morphine occurs, it should be treated with assisted ventilation and low-dose naloxone (0.0050.02 mg kg-1 iv) or nalbuphine (0.03-0.1 mg kg-1 ) to alter the systemic effects respectively (Wetmore & Glowaski 2000).
Cardiovascular depression due to blockade of sympathetic fibers or to the rostral spread of local anaesthetic to the upper four thoracic dermatomes is another adverse effect. The total volume of local anaesthetics injected epidurally should be carefully assessed and the use of volumes exciding the 0.2 ml kg-1 should be avoided (Bosmans et al. 2011). Post-epidural bradycardia and hypotension leading to decreased venous return, poor cardiac output and systemic vascular resistance as a result of extensive sympathetic blockade may be the preliminary causes of cardiac arrest, especially in patients with untreated hypovolemia. Hypotension may be treated by increasing crystalloid fluid administration and decreasing vaporizer concentrations if under general anaesthesia. The treatment may include the administration of vasopressors, such as ephedrine (0.05 mg kg-1 iv bolus) or dopamine (5-10 μg kg-1 CRI) or anticholinergic agents, such as atropine (0.02-0.04 mg kg-1 iv), if bradycardia-induced hypotension is evident (Savvas et al. 2006, Steagall et al. 2017). It is a common belief that hypotension might be prevented by preloading with a crystalloid solution (Wetmore & Glowaski 2000, Jones 2001). Hypotension may also be treated with the intravenous administration of phenylephrine, a potent a1 adrenergic receptor agonist at a continuous rate infusion of 0.5 - 1 μg kg-1 min-1 (Murphy et al. 2020, Cannarozzo et al. 2023).
High sympathetic blockade may also lead to neurological complications including Horner’s syndrome, Shiff- Sherrington like reflexes, muscular twitches, convulsions and coma due to local anaesthetics central nervous system toxicity (Jones 2001, Bosmans et al. 2011). Horner’s syndrome may be induced when local anaesthetics spread to more cranial neurotomes or in the incidence of inadvertent intrathecal or subdural injections (Freire et al. 2010). Muscular twitches in the hind limbs and tail, and myoclonus may occur following epidural or subarachnoid injection of opioids, especially morphine (Kona Boun et al. 2003). The severity of the spasms may be treated after epidural naloxone administration or systemic opioids and sedatives administration. Moreover, local neurotoxicity due to inadvertent placement of the needle in the spinal cord or large nerve roots in the cauda equina may lead to direct nerve damage inducing neural deficits (Wetmore & Glowaski 2000).
Epidural opioids are mainly responsible for a series of side effects. Both epidural morphine and hydromorphone may induce vomiting and nausea which can be effectively treated with the use of maropitant (1mg kg-1 sc) (Troncy et al. 2002). Pruritus has also been encountered following epidural or intrathecal morphine injection. The administration of ondansetron, naloxone, or even low doses of propofol can be an effective treatment to opioid-related pruritus (Troncy et al. 2012). Urinary retention may occur after opioid administration because of the detrusor muscle relaxation that can cause increased capacity and reduced contractility of the urinary bladder (Kona Boun et al. 2003). Urinary retention can be treated with gentle manual bladder expression after surgery and before patient extubation. This will decrease the atony of the detrusor muscle and the discomfort associated with bladder distension (Kona Boun et al. 2003). The placement of a urinary catheter may facilitate continuous emptying until normal function resumes. Other potential complications of epidural opioids, mostly when administered by an epidural catheter, include alopecia or delayed hair growth an ocalizeded cutaneous reaction, all reported with a low incidence (Valverde 2008).These complications can also be seen with the epidural administration of local anaesthetics as well (Guererro et al. 2014).
Conclusion
Epidural anaesthesia and analgesia can be a relatively simple analgesic technique for a variety of surgical procedures including cesarean section, orthopedic hindlimb procedures and soft tissue surgeries caudal to the umbilicus. The epidural administration of local anaesthetics in combination with opioids reduces the anaesthetic and analgesic requirements and provides an effective analgesia with minimum side effects and good quality recovery. Following adequate training, epidural anaesthesia can be a part of a balanced anaesthetic protocol provided in veterinary patients.
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
a: Spinocan®, B. Braun Melsungen AG
Corresponding author:
Tsitsilianou Alexandra
This email address is being protected from spambots. You need JavaScript enabled to view it.
References
- Adami C, Gendron K (2017) What is the evidence? The issue of verifying correct needle position during epidural anaesthesia in dogs. Vet Anaesth Analg 44 (2), 212-218.
- Almeida RM, Escobar A, Maguilnik S (2010) Comparison of analgesia provided by lidocaine, lidocaine-morphine or lidocaine-tramadol delivered epidurally in dogs following orchiectomy. Vet Anaesth Analg 37 (6), 542-549.
- Bartel AK, Campoy L, Martin-Flores M, Gleed RD, Walker KJ, Scanapico CE, Reichard AB (2016) Comparison of bupivacaine and dexmedetomidine femoral and sciatic nerve blocks with bupivacaine and buprenorphine epidural injection for stifle arthroplasty in dogs. Vet Anaesth Analg 43 (4), 435-43.
- Bosmans T, Piron K, Oosterlinck M, Gasthuys F, Duchateau L, Waelbers T, Samoy Y, Van Vynckt D, Polis I (2012) Comparison of analgesic efficacy of epidural methadone or ropivacaine/methadone with or without pre-operative oral tepoxalin in dogs undergoing tuberositas tibiae advancement surgery. Vet Anaesth Analg 39 (6), 618-27.
- Bosmans T, Schauvliege S, Gasthuys F, Duchateau L, Marcilla MG, Gadeyne C, Polis I (2011) Cardiovascular effects of epidural administration of methadone, ropivacaine 0.75% and their combination in isoflurane anaesthetized dogs. Vet Anaesth Analg 38 (5), 494-504.
- Branson KR, Ko JC, Tranquilli WJ, Benson J & Thurmon JC (1993) Duration of analgesia induced by epidurally administered morphine and medetomidine in dogs. J Vet Pharmacol Ther 16 (3), 369-372.
- Campagnol D, Teixeira Neto FJ, Giordano T, Ferreira TH, Monteiro ER (2007) Effects of epidural administration of dexmedetomidine on the minimumalveolar concentration of isoflurane in dogs. Am J Vet Res 68 (12), 1308–18.
- Campagnol D, Teixeira-Neto J, Peccinini R G, Oliveira F A, Al- vaides R K, & Medeiros L Q (2012) Comparison of the effects of peridural or intravenous methadone on the minimum alveolar concentration of isoflurane in dogs. Vet J 192 (3), 311–315.
- Campoy L (2004) Epidural and spinal anaesthesia in the dog. In Pract 26 (5), 262-269. Campoy L & Read M R (2013) In: Small animal regional anesthesia and analgesia. John Wiley & Sons & Blackwell Publishing, Ames, Iowa, USA, p. 288.
- Cannarozzo C J, Araos J, Martin-Flores M (2023) Phenylephrine and norepinephrine increase blood pressure through opposing physiologic mechanisms in isoflurane-anesthetized dogs receiving acepromazine. Am J Vet Res 5, 1-7.
- Cerasoli I, Tutunaru A, Cenani A, Ramirez J, Detilleux J, Balligand M, Sandersen C (2017) Comparison of clinical effects of epidural levobupivacaine morphine versus bupivacaine morphine in dogs undergoing elective pelvic limb surgery. Vet Anaesth Analg 44 (2), 337-345.
- Crystal G J and Salem M R (2012) The Bainbridge and the “reverse” Bainbridge reflexes: history, physiology, and clinical relevance. Anesth Analg 114(3), 520-532.
- DeRossi R, Benites AP, Ferreira JZ, Neto JM, (2009) Hermeto LCEffects of lumbosacral epidural ketamine and lidocaine in xylazine-sedated cats. J S Afr Vet Assoc 80 (2), 79-83.
- Duke T, Caulkett NA, Ball SD, Remedios AM (2000) Comparative analgesic and cardiopulmonary effects of bupivacaine and ropivacaine in the epidural space of the conscious dog. Vet Anaesth Analg 27 (1), 13–21.
- Duque JC, Valadao CA, Farias A, De Almeida RM, Oleskovicz N (2004) Pre-emptive epidural ketamine or S (+) ketamine in post-incisional pain in dogs: a comparative study. Vet Surg 33 (4), 361–367.
- Ferreira JP (2018) Epidural anaesthesia–analgesia in the dog and cat: considerations, technique and complications. UK-Vet Comp Anim 23 (11), 628 – 636.i
- Cavalcanti RL, Noel-Morgan J (2010) Bupivacaine 0.25% and methylene blue spread with epidural anesthesia in dog. Ve Anaest Anal 37 (1), 63–69.
- Ganem EM, Vianna PT (1996) Marques M Neurotoxicity of subarachnoid hyperbaric bupivacaine in dogs. Reg Anaesth 21 (3), 234–238.
- Garcia – Pereira FL (2018) Epidural anesthesia and analgesia in small animal practice: An update. Vet J 242, 24 – 32.
- Gorgi AA, Hofmeister EH, Higginbotham MJ, Kent M (2006) Effect of body position on cranial migration of epidurally injected methylene blue in recumbent dogs. Am J Vet Res 67 (2), 219–21.
- Groban L, Deal DD, Vernon JC, James RL, Butterworth J (2001) Cardiac resuscitation after incremental overdosage with lidocaine, bupivacaine, levobupivacaine, and ropivacaine in anesthetized dogs. Anesth Analg 92(1), 37-43.
- Grubb T, Lobprise H (2020) Local and regional anaesthesia in dogs and cats: Overview of concepts and drugs (Part 1), Vet Med Sc 6 (2), 209–217.
- Kalchofner Guerrero KS, Guerrero TG, Schweizer-Kölliker M, Ringer SK, Hässig M, Bettschart-Wolfensberger R (2014) Incidence of delayed hair re-growth, pruritus, and urinary retention after epidural anaesthesia in dogs. Tierarztl Prax Ausg K Kleintiere Heimtiere 42 (2), 94 – 100.
- Iff I, Moens Y (2008) Two cases of bradyarrhythmia and hypotension after extradural injections in dogs. Vet Anaesth Analg 35 (3), 265-269.
- Iff I, Moens YP (2010) Evaluation of extradural pressure waves and the ‘lack of resistance’ test to confirm extradural needle placement in dogs. Vet J Sep 185 (3), 328–331.
- Jones R S (2001) Epidural analgesia in the dog and cat. Vet J 161(2), 123-131.
- Kamal M, Talaat, SM (2014) Comparative study of epidural morphine and epidural dexmedetomidine used as adjuvant to levobupivacaine in major abdominal surgery. Eg J Anaesth 30 (2), 137–141.
- Kona-Boun JJ, Cuvelliez S, Troncy E (2006) Evaluation of epidural administration of morphine or morphine and bupivacaine for postoperative analgesia after premedication with an opioid analgesic and orthopedic surgery in dogs. J Am Vet Med Assoc 229 (7), 1103-1112.
- Kona-Boun JJ, Pibarot P, Quesnel A (2003) Myoclonus and urinary retention following subarachnoid morphine injection in a dog. Vet Anaesth Analg 30 (4), 257-264.
- Martinez-Taboada F, Redondo JI (2017) Comparison of the hanging-drop technique and running-drip method for identifying the epidural space in dogs. Vet Anaesth Analg 44 (2), 329–336.
- Mastrocinque S, Almeida TF, Tatarunas AC, Imagawa VH, Otsuki DA, Matera JM, Fantoni DT (2012) Comparison of epidural and systemic tramadol for analgesia following ovariohysterectomy. J Am Anim Hosp Assoc 48 (5), 310 – 319.
- Murphy, KM, Rishniw, M, Silverstein, DC (2022) Use of vasopressors for treatment of vasodilatory hypotension in dogs and cats by Diplomates of the American College of Veterinary Emergency and Critical Care. J Vet Emerg Crit Care 32 (6), 714-722.
- O’Hearn AK, Wright BD (2011) Coccygeal epidural with local anesthetic for catheterization and pain management in the treatment of feline urethral obstruction. J Vet Emerg Crit Care 21 (1), 50–52.
- Otero P, Tarragona L, Ceballos M, Portela D (2010) Epidural cephalic spread of a local anesthetic in dogs: a mathematical model using the column length. Vet Anaesth Analg 37. In: Proceedings of the 10th World Congress of Veterinary Anaesthesia 2009 Glasgow, Scotland, p. 35.
- Otero PE, Verdier N, Zaccagnini AS, Fuensalida SE, Tarragona L, Portela DA (2015) The use of a nerve stimulation test to confirm sacrococcygeal epidural needle placement in cats. Vet Anaesth Analg 42 (1), 115–118.
- Pettifer G, Dyson D (2000) Hydromorphone: a cost-effective alternative to the use of oxymorphone. Can Vet J 41(2), 135-137.
- Romano M, Portela DA, Breghi G, Otero PE (2016) Stress-related biomarkers in dogs administered regional anesthesia or fentanyl for analgesia during stifle surgery. Vet Anaesth Analg 43 (1), 44–54.
- Saritas ZK, Saritas TB, Pamuk K, Korkmaz M, Demirkan I, Yaprakci MV, Sivaci RG (2014) Comparison of the effects of lidocaine and fentanyl in epidural anesthesia in dogs. Bratisl Lek Listy 115 (8), 508-13.
- Sasauchi K, Sunada K, Nakamura T (2023) Long-Term Evaluation of Continuous Epidural Anesthesia in an Improved Canine Model. Anesth Pain Med 6 (4), e35458.
- Savvas I, Anagnostou T, Papazoglou LG, Raptopoulos D (2006) Successful resuscitation from cardiac arrest associated with extradural lidocaine in a dog. Vet Anaesth Analg 33 (3), 175-178.
- Smith LJ, Yu JK (2001) A comparison of epidural buprenorphine with epidural morphine for postoperative analgesia following stifle surgery in dogs. Vet Anaesth Analg 28 (2), 87-96.
- Steagall PV, Millette V, Mantovani FB, Gilbert P, Luna SP, Duke-Novakovski T (2009) Antinociceptive effects of epidural buprenorphine or medetomidine, or the combination, in conscious cats. J Vet Pharmacol Ther 32(5), 477-484.
- Steagall PVM, Simon BT, Teixeira Neto FJ, Luna SPL (2017) An update on drugs used for lumbosacral epidural anesthesia and analgesia in dogs. Front Vet Sci 12 (4), 68.
- Torske KE, Dyson DH, Conlon PD (1999) Cardiovascular effects of epidurally administered oxymorphone and an oxymorphonebupivacaine combination in halothane-anesthetized dogs. Am J Vet Res 60, 194-200.
- Torruella X, Potter J, Huuskonen V (2023) Sacrococcygeal epidural administration of 0.5% bupivacaine in seven cats undergoing pelvic or hind limb orthopaedic procedures. Ir Vet J 76 (1), 1.
- Towers T (2020) Comparison of epidural morphine and buprenorphine for hindlimb orthopedic surgery in dogs. Veterinary Evidence 5(2).
- Troncy E, Junot S, Keroack S, Sammut V, Pibarot P, Genevois JP, Cuvelliez S (2002) Results of preemptive epidural administration of morphine with or without bupivacaine in dogs and cats undergoing surgery: 265 cases (1997–1999). J Am Vet Med Assoc 221 (5), 666–672.
- Tyagi A (2017) Thoracic epidural block in sepsis: looking beyond the known. J Anaesthesiol Clin Pharmacol 33 (2), 148-150.
- Valverde A, Skelding A (2019) Comparison of calculated lumbosacral epidural volumes of injectate using a dose regimen based on body weight versus length of the vertebral column in dogs. Vet Anaesth Analg 46 (1), 135-140.
- Valverde A (2008) Epidural analgesia and anesthesia in dogs and cats. Vet Clin North Am Small Anim Pract 38(6), 1205-1230.
- Wetmore LA, Glowaski MM (2000) Epidural analgesia in veterinary critical care. Clin Tech Small Anim Pract 15 (3), 177–188.
- Yoshitomi T, Kohjitani A, Maeda S, Higuchi H, Shimada M, Miyawaki T (2008) Dexmedetomidine enhances the local anesthetic action of lidocaine via an alpha-2A adrenoceptor. Anesth Analg 107 (1), 96-101.



