Kouki Μ.Ι.Veterinarian, MSc, PhD, Private Practitioner
MeSH keywords: feline stomatitis, inflammation, oral cavity, therapy
Abstract
Feline chronic gingivostomatitis (FCG) is a particularly painful disease of the oral cavity of the cat. Affected cats exhibit erosive, ulcerative or hyperplastic lesions at any site of the mucosa that can be accompanied by other dental diseases. Feline gingivostomatitis should be differentiated by other inflammatory conditions of the mouth, such as other types of stomatitis or neoplasms. Aetiology remains elusive, but is considered to arise from an inappropriate immune response to antigenic stimulation. Feline calicivirus also seems to play a role. Therapeutically, various protocols with diverse results have been pursued. Immunosuppressive, immunomodulatory and antinflammatory agents have been tried in clinical practice. However, the clinical picture of cats affected by FCG, is impressively improved after extended extractions. Medical treatment should be administered concomitantly for extended periods, even for life in specific cases.
Feline chronic gingivostomatitis (FCG) is a particularly painful disease of the oral cavity. It is characterized by extended inflammation of the mucosa and can be accompanied by other dental diseases. Feline chronic gingivostomatitis has been previously described as plasmacytic gingivitis-pharyngitis of the cat, plasmacytic stomatitis-pharyngitis, lymphocytic-plasmacytic gingivitis (Williams & Aller 1992), gingivitis-stomatitis-pharyngitis complex, chronic gingivitis-stomatitis (Diehl & Rosychuk 1993), and also as gingivostomatitis (Lyon 2005). The disease was originally described based on the infiltration of the oral mucosa by lymphocytes and plasmacytes as well as on the high concentration of γ-immunoglobulines in the blood of the affected animals (Johnessee & Huritz 1983). Modern literature describes the disease as feline chronic gingivostomatitis (FCG) (Camy et al. 2010). Histological examination constitutes a useful diagnostic tool, but the characteristic clinical appearance combined with medical history should suffice for an accurate diagnosis.
Clinical symptoms
The disease affects animals of both sexes, all breeds and ages (10 months-15 years) with an average age of 8 years (Healey et al. 2007, Hennet et al. 2011). Clinical signs include oral pain or pain upon palpation of the muzzle, halitosis, ptyalism sometimes accompanied by traces of blood, decreased grooming, hyporexia, weight loss, irritability, decreased activity (Winer et al. 2016). Animals may exhibit erosive, ulcerative and/or hyperplastic lesions at any site of the mucosa, such as the gums, lip/buccal and palatal mucosa, the tongue, the molar salivary gland, the palatoglossal arches and the pharynx (Bellows 2010, Lommer 2013) (Figures 1-3). Based on the distribution of the lesions, FCG can be described as (Camy et al. 2010):
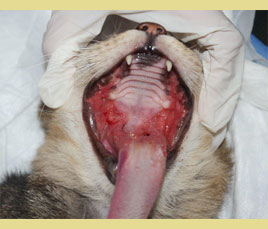
Figure 1. Extensive inflammation with ulcerative and mild hyperplastic lesions of the palatoglossal arches. (source of the images: Maria Kouki, Serafeim Papadimitriou)
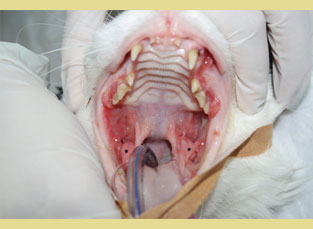
Figure 2. Feline chronic gingivostomatitis. Hyperplastic lesions of the cheeks (white asterisk) and inflammation of the molar salivary glands are characteristic (black asterisk).
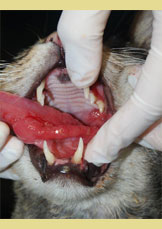
Figure 3. Extensive inflam- mation of the tongue with hyperplastic lesions of the lateral surface (asterisk).
FCG type 1, where the inflammation is mainly located on the gingival and lip/buccal mucosa and FCG type 2, where the inflammation is mainly located on the gingiva of the aboral part of the oral cavity, with or without gingival and/or lips/buccal mucosa inflammation.
Feline chronic gingivostomatitis should be differentiated by other inflammatory conditions of the oral cavity of the cat with similar clinical appearance, such as other types of stomatitis, juvenile periodontitis, eosinophilic granuloma, various metabolic disorders and also neoplasms since therapy and prognosis greatly vary. It is estimated that 30% of the cats affected by FCG do not respond to any treatment (Jennings et al. 2013). Moreover, it has been postulated that the vast majority of cats suffering from FCG also develop esophagitis which is not clinically apparent, probably because it is veiled by the severity of oral cavity symptoms (Kouki et al. 2017). In the same study, squamous epithelium was replaced by metaplastic columnar epithelium in 5 biopsy specimens from the esophagus, while revision endoscopy revealed macroscopic healing of the esophageal mucosa in 2 animals that were FCG free.
Etiology of FCG
Etiology of FCG remains elusive, even though it is generally accepted it is multifactorial in nature with various inciting factors. It seems that FCG results from an improper immune response to oral antigenic stimulation (Lommer & Verstraete 2003, Lommer 2013, Farcas 2014).
Systemic pathogens such as FIV, FeLV, Bartonella και FCV, as well as various dental diseases (tooth resorption, periodontal disease) and hypersensitivity to microbial plaque, food allergy etc. have been implicated (Tenorio et al. 1991, Pedersen1992, Reubel et al. 1992, White et al. 1992, Diehl & Rosychul 1993, Waters et al. 1993, Reubel et al. 1994, Lommer &Verstraete 2003, Lyon 2005, Quimby et al. 2007, Lee 2010, Lommer 2013). Krumbeck et al. (2011) described the potential role of fungi on the etiopathogenesis of the disease. Among the implicated viruses, feline calicivirus is more often investigated but the conflicting results of the studies do not allow for safe results to be drawn concerning its role in the pathogenesis of the disease (Nakanishi et al. 2019, Fried et al. 2021, Fontes et al. 2022). A recent study concluded that cats living in multi-cat households are more prone to develop stomatitis, stressing out the role of infectious agents (Peralta & Carney 2019). To sum up, feline calicivirus seems to play a role in the etiopathogenesis of FCG which is yet to be elucidated.
The oral bacterial flora of cats with FCG has been identified with culture dependent and culture independent methods. It was found that the microbial diversity significantly decreases in cats with FCG in which the predominant species is P. multocida subs. Multocida and 4,7 % of the identified species were classified as novel (Dolieslager et al. 2011). Moreover, according to Dolieslger et al. (2013), Tanerella forsythiaarises extended immune response on behalf of the host. The synergistic mechanism of action between the two microorganisms has been proven (Sharma 2010). On the other hand, Capnocytophaga canimorsus represented the predominant species among healthy cats and 43.7 % were identified as novel (Dolieslager al. 2011, Dolieslager et al. 2013). The infiltration of the gingiva with high numbers of mast cells, suggests the notion that they may play a role in the shift of the microbial flora in cats with FCG (Arzi et al 2010). However, Rodrigues et al. (2019) found higher bacterial diversity in cats suffering from periodontitis or FCG, with Bacteroides phylum being the most abundant, highlighting the important role played by the bacterial biofilm. Nevertheless, researchers conclude that the microbial flora of FCG cats is mainly constituted by gram-negative anaerobic microorganisms (Rodrigues et al. 2019). The contribution of the microbial plaque is also stressed out by Hennet (1995) and Bellei et al. (2008).
According to the aforementioned studies, the clinical appearance of FCG cats is greatly improved following extensive dental extractions That way, additional microbial plaque built up is prevented. Finally, stress and food allergens have been incriminated (Williams & Aller 1991, Diehl & Rosychuk 1993, Lommer 2013).
Concerning the immunological background of the disease, affected cats exhibit elevated concentrations of immunoglobulins in the blood serum and polyclonal hypergammaglobulinemia (Johnessee & Hurvitz 1983, White et al. 1992). Zetner et al. (1989), found elevated concentrations of G, M, and A immunoglobulins (IgG, IgM, IgA) in the blood serum of cats with chronic inflammatory conditions of the oral cavity. Another study showed that cats with chronic gingivostomatitis had significantly higher salivary concentrations of IgG, IgM and albumin, and higher serum concentrations of IgG, IgM and IgA, but significantly lower salivary concentrations of IgA than their healthy counterparts (Harley et al. 2003). Evidence on the immunological basis of the condition is further suggested by the descriptions of the histopathological changes and blood phenotype of the affected cats. A previous study on the immunohistochemical characterization of oral mucosal lesions, detected numerus T (including CD4+ and CD8+ cells) and B lymphocytes, mast cells in the lamina propria and submucosa with CD8+ cells predomination over CD4+ (Harley et al. 2011). Significant increase in mRNA toll-like receptors and proinflammatory genes encoding TNF-a, IFN-γ, IL-1β, and IL-6 were found in tissue biopsies obtained from FCG cats (Dolieslager et al. 2013). Vapniarsky et al. (2020), found that the oral mucosal tissues from cats with FCG had high tissue infiltration of B and T cells including both CD4+ and CD8+ lymphocytes and cells positive for CD25 and FOXP3 and mixed gene populations associated with inflammatory signaling pathways. Respectively, high CD8+ T lymphocytes and other proinflammatory cytokines have been detected in the blood of FCG cats (Arzi et al. 2016, Arzi et al. 2017, Arzi et al. 2020).
In conclusion, the cause of FCG remains elusive, although inappropriate immune response in underlying infectious agents, such as bacteria and some viruses has been implicated.
Treatment of FCG
Therapeutically, surgical and medical approaches have been proposed. However, medical treatment alone does not have favorable long-term results, constituting the surgical approach, the gold standard (Lee et al. 2020). Regardless of modality, the importance of analgesic therapy cannot be overemphasized (Lee et al. 2020). Long-term administration of opiates such as buprenorphine has been proven both safe and effective (Stathopoulou et al.). Meanwhile, new “therapeutic targets” for treating FCG, like the endocannabinoid system have been emphasized (Polidoro et al. 2020). In general, medical therapies exhibit various outcomes due to the multifactorial nature of the disease.
Diagnosis and treatment of FCG, can be troubling and bring the clinician to a therapeutic end. The cat owner should be informed that the ultimate target of therapy is to adequately control relapses and assure a substantial quality of life rather than complete remission. Leading treatment modalities are discussed below.
Surgical treatment
Four published studies have investigated the effectiveness of tooth extraction in FCG cats (Hennet 1997, Bellei et al. 2008, Jennings et al. 2015, Druet & Hennet 2017). A substantial improvement or resolution in 80% the FCG cases, and little or no improvement in 20% of the cases has been shown in two studies (Hennet 1997, Bellei et al. 2008). Jennings et al. (2015) revealed that 28.4% of cats achieved complete resolution, 39% achieved substantial clinical improvement, 26.3% had little improvement, and 6.3% had no improvement following dental extractions. Finally, in a study of Druet and Hennet (2017), 51.8% achieved clinical cure or significant improvement within a median time of 38 days. Partial-mouth extraction plus extraction of other teeth that have indication for extraction, such as periodontitis, fracture, resorption, root retention is the evidence-based recommendation for surgical management. In case there is no positive response within 1-4 months after partial-mouth extractions, full-mouth extractions should be performed (Lee et al. 2020). Finally, cauterization with CO2 laser also seems to be beneficial (Lewis et al. 2007) (Figure 4).
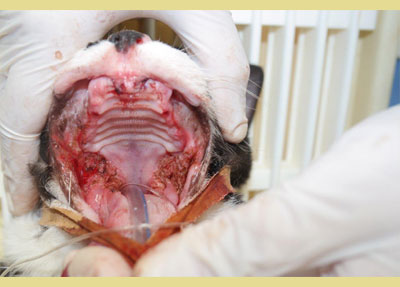
Figure 4. CO2 laser cauterization in a refractory case.
Medical management
Besides surgical management, adjuvant medical management, sometimes lifelong, is deemed necessary in most FCG cases. As already mentioned, FCG is an immune-mediated inflammatory disease, the therapy of which is based on fighting anaerobic bacteria, and immunosuppression (Winer et al. 2016). Proposed medicaments for FCG involve mainly antimicrobials and non-steroidal antinflammatory drugs, steroids, recombinant interferon -ω, cyclosporin and more recently mesenchymal stem cells.
Medical management is included in all treatment protocols that have been tested, without prior swabbing for cultivation and antibiogram (Krumbeck et al. 2021). Commonly used antibiotics include clindamycin, amoxicillin, amoxicillin with clavulanic acid, metronidazole and doxycycline (Frost & Williams, 1986, Harvey 1991, Harvey 1994, Lyon 2005, Wiggs 2007). Dolislager (2012) also suggests cefovecin based on her study results.
When dealing with chronic and acute post-surgical pain, non-steroidal antinflammatory drugs constitute the cornerstone (Epstein et al. 2015). According to the literature, none of the veterinary approved non-steroidal antinflammatory drugs has been associated with greater risk for side effects (Kukanish et al. 2012). Meloxicam is been approved in many countries worldwide. On the other hand, corticosteroids have been traditionally considered to be the holy grail for treating immune mediated diseases. Ergo they have been empirically used widely in FCG. Nonetheless, the clinical outcome and duration of their potential benefit has been minimally studied. A study concerning calicivirus positive cats with refractory FCG, prednisolone was used in the control group as opposed to a group treated with recombinant interferon Ω (Ueda et al. 1993). Three out of 11 cats of the study treated with prednisolone (23%) were significantly improved (Ueda et al. 1993). Even though it is a common belief that cats are resistant to corticosteroid side effects, their administration should be closely monitored as long-term use has been linked to serious complications (Smith et al. 2004, Lowe et al. 2006, Campbell & Graves 2008, Nerhagen 2021). In general, oral or parenteral formulations are preferred over repositol corticosteroids because there is less prolonged suppression of the hypothalamic-pituitary-adrenal axis, a greatly enhanced ability to monitor and adjust the dose, and less pronounced side effects (Feldman & Nelson 2004). The use of repositol corticosteroids should be reserved for those cats in which oral dosing is not possible due to patient or owner compliance (Lowe et al. 2008).
Feline recombinant interferon -ω (rFeIFN-ω) has been studied for its efficacy in FCG. rFeIFN-ω has been traditionally used for its antiviral properties (Ueda et al. 1993) and is believed to promote immunoregulation via lymphoid tissues (Schellekens et al. 2001, Cummins et al. 2005). After 3 months of transmucosal administration of rFeIFN-ω in 19 cats, 45% of the animals showed clinical remission while 10% of which were clinically cured (Ueda et al. 1993). However, the study concludes that the use of rFeIFN-ω does not outweight the use of prednisolone. Matsumoto et al. (2018), stated that the subcutaneous injection of rFeIFN-ω in FCG cats that are co-infected with calicivirus may be of benefit.
Cyclosporin A (CsA) is an immunosuppressant which has been used in FCG treatment protocols. CsA is involved mostly in the cell-mediated rather than the humoral immunity. Very few studies have investigated the mechanism of action of cyclosporin in cats. Nonetheless, CsA suppresses in vitro the lymphoblast transformation, and mRNA expression for IL-2, IL-4, IL-10, GM-CSF, IFN-γ, TNF-α, and lymphocytes releasing IL-2 in a dose dependent manner in the peripheral blood (Colombo & Sartori 2018). It may also have inhibitory effects on B-cell reproduction (Winslow et al. 2006). The efficacy of CsA in FCG has been reported in two studies. Vercelli et al. (2002), examined the efficacy of oral cyclosporine in 8 cats not previously treated with extractions, half of which (50%) were reported to achieve clinical remission, whereas the rest had partial to fairly good improvement. In another long-term clinical trial oral cyclosporine was administered to 9 cats that had previously been treated with extractions, there was a statistical significance in the number of cats experiencing significant clinical improvement (77.8%). At the end of the 6-week observation period, there was a statistically significant difference among cats with whole-blood cyclosporine levels > 300 ng/ml (72.3 % improvement) compared with cats with cyclosporine levels <300 ng/ml (28.2 % improvement) (Lommer 2013).
In the context of immunomodulation, mesenchymal stem cells (MSC) have been administered in cats with refractory FCG. Mesenchymal stems cells play an important role in the regulation of the immune system. They produce various antinflammatory and proinflammatory cytokines, chemocines, and prostaglandines. They are considered to be safe with minimal or no mutagenic effect and can be used for revitalizing or repairing damaged tissues (Dias et al. 2019). Their immunomodulatory effect has been studied in inflammatory and immune mediated diseases of the cat such as FCG, acute and chronic renal disease, enteropathies and asthma (Quimby & Borjesson 2018). The efficacy of both autologous and allogenic, fresh, adipose-derived MSCs administered intravenously has been studied in cats with refractory FCG (Arzi et al. 2016, Arzi et al. 2017). Treatment with autologous adipose-derived MSCs in 7 cats resulted in a positive response rate reflected by clinical remission in 42.8%, and substantial improvement in 28.6% of the cats (Arzi et al. 2016) compared to treating with allogenic MSCs (28.6% achieved clinical remission, 28.6% achieved substantial improvement) (Arzi et al. 2017). In both clinical trials, all cats had undergone full-mouth extractions, while administration of stem cells without prior extraction failed.
Summary
FCG constitutes a particularly painful, multifactorial immune-mediated disease, possibly potentiated or exacerbated by viral infection. The current first-line of treatment involves tooth extractions with or without concurrent medical treatment. New modalities of treatment for the control of inflammation and pain ought to be sought to replace the current sometimes life-long required medical treatments.
Conflict of interest
The author declares that there is no conflict of interest.
References
- Arzi B, Clark KC, Sundaram A, Spriet M, Verstraete FJM, Walker NJ, Loscar MR, Fazel N, Murphy WJ, Vapniarsky N, Borjesson DL (2017) Therapeutic efficacy of fresh, allogeneic mesenchymal stem cells for severe refractory feline chronic gingivostomatitis. Stem Cells Transl Med 6, 1710–22.
- Arzi B, Mills-Ko E, Verstraete FJ, Kol A, Walker NJ, Badgley MR, Fazel N, Murphy WJ, Vapniarsky N, Borjesson DL (2016) Therapeutic efficacy of fresh, autologous mesenchymal stem cells for severe refractory gingivostomatitis in cats. Stem Cells Transl Med 5, 75–86.
- Arzi B, Peralta S, Fiani N, Vapniarsky N, Taechangam N, Delatorre U, Clark KC, Walker NJ, Loscar MR, Lommer MJ, Fulton A, Battig J, Borjesson DL (2020) A multicenter experience using adipose-derived mesenchymal stem cell therapy for cats with chronic, non-responsive gingivostomatitis. Stem Cell Res Ther 11, 115.
- Arzi Β, Murphy B, Cox DP, Vapniarsky N, Kass PH, Verstraete FMJ (2010) Presence and quantification of mast cells in the gingiva of cats with tooth resorption, periodontitis and chronic stomatitis. Arch Oral Biol 55, 148-154.
- Belgard S, Truyen U, Thibault JC, Sauter-Louis C, Hartmann K (2010) Relevance of feline calicivirus, feline immunodeficiency virus, feline leukemia virus, feline herpesvirus and Bartonella henselae in cats with chronic gingivostomatitis. Berl Munch Tierarztl Wochenschr 123, 369–76.
- Bellei E, Dalla F, Masetti L, Pisoni L, Joechler M (2008) Surgical therapy in chronic feline gingivostomatitis (FCGS). Vet Res Commun 32, S231–4.
- Bellows J (2010) Oral pathology. In: J Bellows ed. Feline dentistry: oral assessment, treatment, and preventative care. 1st ed. Blackwell Iowa, pp.101-148.
- Camy G, Fahrenkrug P, Gracis M, Hennet P, Johnston N, Mihaljevic S, Schreyer J (2010) Proposed guidelines on the management of Feline Chronic Gingivostomatitis (FCGS) syndrome: a consensus statement. Consultation version, September 2010, Nice France. Colombo S, Sartori R (2018) Ciclosporin and the cat: Current understanding and review of clinical use. J Feline Med Surg 20,244-255.
- Cummins JM, Krakowka GS, Thompson CG (2005) Systemic effects of interferons after oral administration in animals and humans.Am J Vet Res 66, 164–76.
- Dias IE, Pinto PO, Barros LC, Viegas CA, Dias IR, Carvalho PP (2019) Mesenchymal stem cells therapy in companion animals: useful for immune-mediated diseases? BMC Vet Res 15, 358.
- Diehl K, Rosychuk RA (1993) Feline gingivitis-stomatitis-pharyngitis. Vet Clin North Am Small Anim Pract 23, 139–53.
- Dolieslager SM, Lappin DF, Bennett D, Graham L, Johnston N, Riggio MP (2013) The influence of oral bacteria on tissue levels of Toll-like receptor and cytokinemRNAs in feline chronic gingivostomatitis and oral health. Vet ImmunolImmunopathol 151, 263–74.
- Dolieslager SMJ, Bennett D, Johnston N, Riggio MP (2013) Novel bacterial phylotypes associated with the healthy feline oral cavity and feline chronic gingivostomatitis. Res Vet Sci 94, 428-432.
- Dolieslager SMJ, Lappin DF, Bennett D, Graham L, Johnston N, Riggio MP (2013) The influence of oral bacteria on tissue levels of Toll-like receptor and cytokine mRNAs in feline chronic gingivostomatitis and oral health. Vet Immunol Immunopathol 151, 263-274.
- Dolieslager SMJ, Riggio MP, Lennon A, Lappin DF, Johnston N, Taylor D, Bennett D (2011) Identification of bacteria associated with feline chronic gingivostomatitis using culture dependent and culture-independent methods. Vet Microbiol 148, 93-98.
- Dolislager SMJ (2012) Studies on the aetiopathogenesis of feline chronic gingivostomatitis college of medicine.Veterinary and Life Sciences University of Glasgow. Dowers KL, Hawley JR, Brewer MM, Morris AK, Radecki SV, Lappin MR (2010) Association of Bartonella species, feline calicivirus, and feline herpesvirus 1 infection with gingivostomatitis in cats.J Feline Med Surg 12, 314–21.
- Druet I, Hennet P. Relationship between feline calicivirus load, oral lesions, and outcome in feline chronic gingivostomatitis (caudal stomatitis): retrospective study in 104 cats. Front Vet Sci 2017, 209. Epstein ME, Rodanm I, Griffenhagen G, Kadrlik J, Petty MC, Robertson SA, Simpson W; AHAA; AAFP (2015) AAHA/AAFP pain management guidelines for dogs and cats. J Feline Med Surg 17, 251-72.
- Farcas N, Lommer MJ, Kass PH, Verstraete FJM (2014) Dental radiographic findings in cats with chronic gingivostomatitis (2002-2012). J Am Vet Med Assoc 244, 339-45.
- Feldman EC, Nelson RW (2004) Glucocorticoid therapy. In: Feldman EC, Nelson RW, eds. Canine and Feline Endocrinology and Reproduction. 3rd ed. W.B. Saunders St. Louis pp. 464-83.
- Fontes C, Vieira MC, Oliveiray M, Lourenco L, Requicha J, Viegas C, FaÍsca P, Seixas F, Pires MA (2022) Calicivirus and NK cells in chronic feline gingivostomatitis. What else? Comparative Pathology 191, 27.
- Fried WA, Soltero-Rivera M, Ramesh A, Lommer MJ, Arzi B, DeRisi JL, Horst JA (2021) Use of unbiased metagenomic and transcriptomic analyses to investigate the association between feline calicivirus and feline chronic gingivostomatitis in domestic cats. Am J Vet Res 82, 381-394.
- Frost P, Williams CA (1986) Feline dental disease. Vet Clin North Am Small Anim Pract 16, 851-873.
- Harley R, Gruffydd-Jones TJ, Day MJ (2003) Salivary and serum immunoglobulin levels in cats with chronic gingivostomatitis. Vet Rec 152, 125-129.
- Harley R, Gruffydd-Jones TJ, Day MJ (2011) Immunohistochemical characterization of oral mucosal lesions in cats with chronic gingivostomatitis. J Comp Path 144, 239-250.
- Harvey CE (1991) Oral inflammatory diseases in cats. Journal of the American Animal Hospital Association 27, 585-591.
- Harvey CE (1994) Oral and Dental Diseases. In: G Sherding, ed. The Cat: Diseases and clinical management. W.B. Saunders Philadelphia, p. 1117-1151
- Healey KA, Dawson S, Burrow R, Cripps P, Gaskell CJ, Hart CA, Pinchbeck GL, Radford AD, Gaskell RM (2007) Prevalence of feline chronic gingivostomatitis in first opinion veterinary practice. J Feline Med Surg 9, 373-381.
- Hennet P (1997) Chronic gingivo-stomatitis in cats: long-term follow-up of 30 cases treated by dental extractions. J Vet Dent 14, 15–2.
- Hennet PR, Camy GA, McGahie DM, Albouy MV (2011) Comparative efficacy of a recombinant feline interferon omega in refractory cases of calicivirus-positive cats with caudal stomatitis: a randomised, multi-centre, controlled, double-blind study in 39 cats. J Feline Med Surg 13, 577-587.
- Jennings MW, Lewis JR, Soltero-Rivera MM, Brown DC, Reiter AM (2015) Effect of tooth extraction on stomatitis in cats: 95 cases (2000-2013). J Am Vet Med Assoc 246, 654-60.
- Jennings MW, Lewis JR, Soltero-Rivera MM, Brown DC, Reiter AM (2015) Effect of tooth extraction on stomatitis in cats: 95 cases (2000-2013). J Am Vet Med Assoc 246, 654-60.
- Johnessee J, Huritz A (1983) Feline plasma cell gingivitis-pharyngitis. J Am Anim Hosp Assoc 19, 179-181.
- Kouki MI, Papadimitriou SA, Psalla D, Kolokotronis A, Rallis TS (2017) Chronic gingivostomatitis with esophagitis in cats. J Vet Intern Med 31, 1673–9.
- Krumbeck JA, Reiter AM, Pohl JC, Tang S, Kim YJ, Linde A, Prem A, Melgarejo T (2021) Characterization of Oral Microbiota in Cats: Novel Insights on the Potential Role of Fungi in Feline Chronic Gingivostomatitis. Pathogens 10, 904.
- KuKanich B, Bidgood T, Knesl O (2012) Clinical pharmacology of nonsteroidal anti-inflammatory drugs in dogs. Vet Anaesth Analg 39, 69–90.
- Lee DB, Verstraete FJM, Arzi B (2020) An Update on Feline Chronic Gingivostomatitis. Vet Clin North Am Small Anim Pract 50, 973-982.
- Lee M, Bosward KL, Norris JM (2010) Immunohistological evaluation of feline herpesvirus-1 infection in feline eosinophilic dermatoses or stomatitis.J Feline Med Surg 12, 72–9.
- Lewis JR, Tsugawa AJ, Reiter AM (2007) Use of CO2 laser as an adjunctive treatment for caudal stomatitis in a cat. J Vet Dent 24, 240-9.
- Lien YH, Huang HP, Chang PH. Iatrogenic hyperadrenocorticism in 12 cats (2006) J Am Anim Hosp Assoc 42, 414-23.
- Lommer MJ (2013) Efficacy of cyclosporine for chronic, refractory stomatitis in cats: a randomized, placebo-controlled, double-blinded clinical study. J Vet Dent 30, 8–17.
- Lommer MJ (2013) Oral inflammation in small animals. Vet Clin North Am Small Anim Pract, 43, 555-571.
- Lommer MJ, Verstraete FJM (2003) Concurrent oral shedding of feline calicivirus and feline herpesvirus 1 in cats with chronic gingivostomatitis Oral Microbiol Immunol 18, 131–4.
- Lowe AD, Campbell KL, Graves T (2008) Glucocorticoids in the cat. Vet Dermatol 19, 340-7.
- Lyon KF (2005) Gingivostomatitis. Vet Clin North Am Small Anim Pract 35, 891–911.
- Matsumoto H, Teshima T, Iizuka Y, Sakusabe A, Takahashi D, Amimoto A, Koyama H (2018) Evaluation of the efficacy of the subcutaneous low recombinant feline interferon-omega administration protocol for feline chronic gingivitis-stomatitis in feline calicivirus-positive cats. Res Vet Sci 121, 53–8.
- Nakanishi H, Furuya M, Soma T, Hayashiuchi Y, Yoshiuchi R, Matsubayashi M, Tani H, Sasai K (2019) Prevalence of microorganisms associated with feline gingivostomatitis. J Feline Med Surg 21, 103-108.
- Nerhagen S, Moberg HL, Boge GS, Glanemann B (2021) Prednisolone-induced diabetes mellitus in the cat: a historical cohort. J Feline Med Surg Feb 23, 175-180.
- Pedersen NC (1992) Inflammatory oral cavity diseases of the cat. Vet Clin North Am Small Anim Pract 22, 1323–45.
- Peralta S, Carney PC (2019) Feline chronic gingivostomatitis is more prevalent in shared households and its risk correlates with the number of cohabiting cats. J Feline Med Surg 21, 1165–71.
- Polidoro G, Galiazzo G, Giancola F, Papadimitriou S, Kouki M, Sabattini S, Rigillo A, Chiocchetti R (2021) Expression of cannabinoid and cannabinoid-related receptors in the oral mucosa of healthy cats and cats with chronic gingivostomatitis. J Feline Med Surg 23, 679-691.
- Quimby JM, Borjesson DL (2018) Mesenchymal stem cell therapy in cats: Current knowledge and future potential. J Feline Med Surg 20, 208-216.
- Quimby JM, Elston T, Hawley J, Brewer M, Miller A, Lappin MR (2007) Evaluation of the association of Bartonella species, feline herpesvirus 1, feline calicivirus, feline leukemia virus and feline immunodeficiency virus with chronic feline gingivostomatitis. J Feline Med Surg 10, 66–72.
- Reubel GH, George JW, Higgins J, Pedersen NC (1994) Effect of chronic feline immunodeficiency virus infection on experimental feline calicivirus-induced disease. Vet Microbiol 39, 335–51.
- Reubel GH, Hoffmann DE, Pedersen NC (1992) Acute and chronic faucitis of domestic cats. A feline calicivirus-induced disease. Vet Clin North Am Small Anim Pract 22, 1347–60.
- Rodrigues MX, Bicalho RC, Fiani N, Lima SF, Peralta S (2019) The subgingival microbial community of feline periodontitis and gingivostomatitis: characterization and comparison between diseased and healthy cats. Sci Rep 9, 12340.
- Schellekens H, Geelen G, Meritet JF, Maury C, Tovey MG (2001) Oromucosal interferon therapy: relationship between antiviral activity and viral load. J Interferon Cytokine Res 21, 575–581
- Sharma A (2010) Virulence mechanisms of Tannerella forsythia. Periodontol 2000, 54, 106-116.
- Smith SA, Tobias AH, Fine DM, Jacob KA, Ployngam T (2004) Corticosteroid-Associated Congestive Heart Failure in 12 Cats. Intern J Appl Res Vet Med 2, 159-170.
- Stathopoulou TR, Kouki MI, Pypendop BH, Johnston A, Papadimitriou SA, Pelligand L (2018) “Evaluation of analgesic effect and absorption of buprenorphine after buccal administration in cats with oral disease”. J Fel Med Surg 20, 704-710.
- Tenorio AP, Franti CE, Madewell BR, Pedersen NC (1991) Chronic oral infections of cats and their relationship to persistent oral carriage of feline calici-, immunodeficiency, or leukemia viruses. Vet Immunol Immunopathol 29, 1–14.
- Ueda Y, Sakurai T, Kasama K, Satoh Y, Atsumi K, Hanawa S, Uchino T, Yanai A (1993) Pharmacokinetic properties of recombinant feline interferon and its stimulatory effect on 2’,5’-oligoadenylate synthetase activity in the cat. J Vet Med Sci 55, 1–6.
- Vapniarsky N, Simpson DL, Arzi B, Taechangam N, Walker NJ, Garrity C, Bulkeley E, Borjesson DL (2020) Histological, Immunological, and Genetic Analysis of Feline Chronic Gingivostomatitis. Front Vet Sci 7, 310.
- Vercelli A, Raviri G, Cornegliani L (2006) The use of oral cyclosporin to treat feline dermatoses: a retrospective analysis of 23 cases. Vet Dermatol 17, 201–6.
- Waters L, Hopper CD, Gruffydd-Jones TJ, Harbour DA (1993) Chronic gingivitis in a colony of cats infected with feline immunodeficiency virus and feline calicivirus Vet Rec 132, 340–2.
- White S, Rosychuk RAW, Janik TA, Denerolle P, Schultheiss P (1992) Plasma cell stomatitis gingivitis in cats: 40 cases (1973-1991). J Am Vet Med Assoc 200, 1377-1380.
- White SD, Rosychuk RA, Janik TA, Denerolle P, Schultheiss P (1992) Plasma cell stomatitis-pharyngitis in cats: 40 cases (1973-1991). J Am Vet Med Assoc 9, 1377–80.
- Wiggs RB (2007) Lymphocytic plasmactic stomatitis. In: GD Norsworthy, ed. The Feline Patient.3rd ed. Blackwell Iowa. Winer JN, Arzi B, Verstraete FJM (2016) Therapeutic management of feline chronic gingivostomatitis: a systematic review of the literature. Front Vet Sci 3, 54.
- Winslow MM, Gallo EM, Neilson JR, Crabtree GR (2006) The calcineurin phosphatase complex modulates immunogenic B cell responses. Immunity 24, 141–52.
- Zetner K, Kampfer P, Lutz H, Harvey C (1989) Comparative immunological and virological studies of chronic oral diseases in cats. Wien Tierarztl Monat 76, 303-308.
Corresponding author:
Maria Kouki
e-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.




