Remember how...
Τissue handling by the practitioner; from collection to submission of the sample to the histopathology lab
> Introduction
Proper handling of tissue samples from their collection to their submission to the histopathology lab is crucial, so that autolysis (self-digestion) and technical errors, that would render the diagnosis difficult or even impossible, can be avoided. Furthermore, the information provided by the practitioner sometimes contributes decisively to the interpretation of the histopathological findings. In any case, the best possible result is achieved when there is collaboration between the practitioner and the histopathologist, implying a common frame of reference in communication. Below is detailed information about the appropriate fixation/ preservation and submission/shipping of tissue samples that are obtained for histopathological examination.
> Tissue fixation
Appropriate and adequate fixation of the tissues is required for an efficient histological observation.
- It prevents autolysis and bacterial decomposition.
- It stabilizes tissue architecture of the original living state.
- It hardens the soft tissues, facilitating further processing.
- It maintains or even enhances the properties of tissue that are required for subsequent biological and chemical staining.
In order to achieve the above, the tissue should be placed in the fixative immediately after its collection.
The choice of fixative is determined by the purpose for which the tissue will be processed and stained or preserved. Neutral formalin solution (see below) is the most commonly used fixative because it allows further staining of tissue with a variety of reagents.
Fixation requires the use of chemical or physical agents such as:
- Aldehydes e.g. formaldehyde, glutaraldehyde.
- Oxidizing agents e.g. osmium tetraoxide, potassium permanganate.
- Chemical protein denaturating agents (coagulants) e.g. acetic acid, methanol, ethanol.
- Physical agents e.g. heating, microwaving, freezing.
- Other agents e.g. mercuric chloride, picric acid.
Factors affecting fixation:
- Hydrogen ion concentration: Optimum fixation pH: 6-8.
- Temperature (T): fixation at room temperature is sufficient (for electron microscopy the appropriate temperature for fixation is 0-4oC).
- Penetrance: The penetrance of common fixatives is slow or limited. If specimens are very large the fixative cannot penetrate and reach the center of the sample. As a result autolysis may occur in the center of thick tissues.
- Osmolarity.
- Concentration of fixative: The most commonly used fixative is neutral formalin 10%. (see: fixation “in practice”).
- Duration of fixation: The fixation time required depends upon the size of the sample, the nature of the tissue and the type of the fixative. Standard fixation time is 24-48 hours. Caution: over fixation of tissue in formalin causes its shrinkage and impairs tissue reactivity.
- Volume ratio of fixative to tissue: The fixative volume should be at least ten times the tissue volume.
> In practice
Fixation
1.When the tissue is collected for routine histological evaluation, the most commonly used fixative is formalin 10%. To achieve the above concentration the commercial product “formol” or “formalin” has to be diluted with distilled water in 1:10.
10% formalin solution
Formol (37-40% Formaldehyde) 100 ml
Distilled water 900ml
It is noted that the commercially used term “formol” refers to a solution of about 37-40% formaldehyde gas in water with the addition of 10-15% methanol stabilizer to suppress polymerization.
If possible, it is best to use neutral buffered formalin for the fixation of the sample.
10% Neutral buffered formalin
Formol (37-40% Formaldehyde) 100ml
Distilled water 900ml
Sodium dihydrogen phosphate Dihydrate (NaH2PO4.2H2O) 4 g
Di-sodium hydrogen phosphate anhydrous (Na2HPO4) 6,5 g
2. When biopsy is performed for research purposes, it is imperative that the tissue is fixed and maintained in neutral buffered formalin solution. It is critical to avoid prolonged fixation, because this would cause shrinkage of the tissue and destruction of certain antigens.
3. When the tissue is collected for immunofluorescence or in case that immunohistochemical examination may be performed after the histopathological evaluation (e.g. in case of T- or B-cell lymphoma, detection of viral antigens or indicators of malignancy etc), the practitioner should contact the histopathology lab for determination of the fixation agent, since some antigens are destroyed in formalin-fixed tissues, while others can be detected only in cryosections. In such cases, it is again critical to avoid prolonged fixation because it impairs tissue reactivity.
4. If the histopathological examination will be performed in frozen sections, the tissue must be frozen in liquid nitrogen. For this purpose, the tissue is wrapped in aluminum foil and immersed in liquid nitrogen for one minute. Then it must be kept frozen (T: -60 to -80 ⁰C) until it is admitted to the laboratory.
5. If the tissue is going to be examined using electron microscopy, it should be fixed and maintained in glutaraldehyde 4% or paraformaldehyde 10%. The above solutions are stored under refrigeration (T:~4⁰C).
6. In cases of “emergency biopsy” (performance of the examination intra-operatively) the tissue is sliced into thin pieces and is fixed in special alcohol solution e.g. Clark’s solution or Carnoy’s fixative or it is fixed by freeze fixation or microwave heating.
Clark’s solution
Absolute alcohol 75 ml
Glacial acetic acid 25 ml
Carnoy’s fixative
Absolute alcohol 60 ml
Chloroform 30 ml
Glacial acetic acid 10 ml
> Comments regarding the management of the fixative solutions and the choice of the tissue containers
- Tissues must be fixed immediately after collection, in order to avoid autolysis. When immediate fixation is not possible, tissues should be preserved in humid conditions (wrapped in gauze soaked with saline) and placed as soon as possible in the fixative.
- Containers used for fixation and shipping of samples must be wide-mouthed because fixation makes tissues firm and inelastic and hus impossible to be taken out of a narrow container after they are fixed.
- If the formalin solution develops white flocs, this means it has lost its efficiency and should be discarded.
- Fixatives (aldehydes → formol) should be handled with caution because they are toxic. They should not be inhaled and skin and mucous-contact should be avoided. In case of an accident the affected area should be rinsed with water and in more severe cases emergency help should be sought by contacting the poison control center.
> Tissue handling
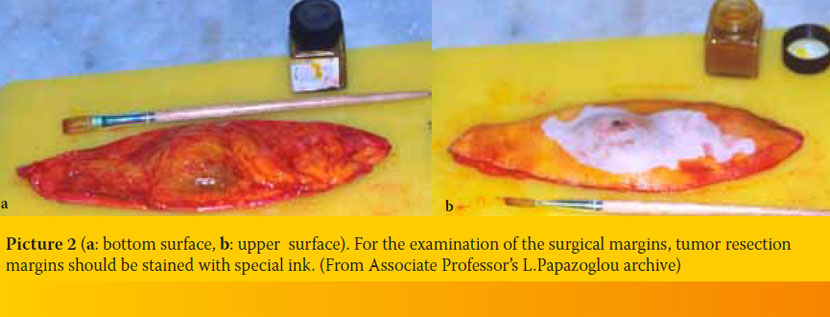
- As previously mentioned, the penetrance of fixatives is limited. Thus, fixation, especially in the center of large specimens could be inadequate and as a result autolysis could continue. For proper fixation, specimens should be less than 1-2 cm thick (ideally less than 0.5 cm thick). However, it is important that the pathologist has an overall view of the whole tissue that has been removed, so that he can choose himself the tissue samples that will be examined; thus, it is preferable for large samples to be deeply incised to facilitate penetration, without being completely dissected (Picture 1a, b). This is particularly important in the cases of neoplasms, in which the pathologist, by examining the surgical margins of the tissue, has to determine if the neoplasm has been completely excised. Ideally, in cases that surgical margins examination is required, tumor resection margins should be stained with special ink (Picture 2a, b). Thus, in case of tissue dissection, recognition of margins is possible.

- When the specimen is extremely large or involves a whole organ (e.g. spleen, mammary gland) it might have to be dissected before its shipment. In such cases, it is important that a photo or a drawing showing tissue original size and anatomical site is sent along with the sample.
- When the specimen is extremely small (bone marrow, transcutaneous liver biopsy, kidney biopsy, endoscopic biopsies of the nasal or gastric mucosa etc) it is recommended that it is placed in the fixative wrapped in filter paper or within biopsy cassettes (Picture 3a, b, c, d).

- If the tissue samples are covered with blood, it is recommended that they are rinsed with saline before being placed in the fixative, because blood delays fixation. Alternatively, it is recommended that the formalin is discarded and replaced after 24 hours.
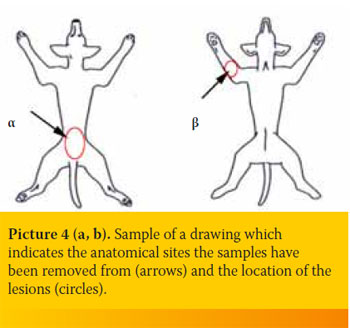
- Skin biopsies should be shipped along with an image showing the exact anatomical site they have been removed from and the location of the lesions (Picture 4a, b).
> Tissues that require special handling
Bones
Formalin is used for bone fixation, as in other tissues. After approximately two days in formalin bones are demineralized in the histopathology lab.
For the histological examination of bone tissue from an amputated limb, skin and surrounding soft tissues should be removed before the sample is collected. If this dissection cannot be carried out right away in the operating room, the amputated limb should be stored under refrigeration (4 ⁰C) until the removal of the surrounding tissues.
Uterus
In cases of ovariohysterectomy the whole uterus is shipped for histological evaluation. The two horns must be incised only on the lateral surface so that they remain attached to the body of uterus. The uterus of large animals should be incised per 1-1,5cm.
Intestine
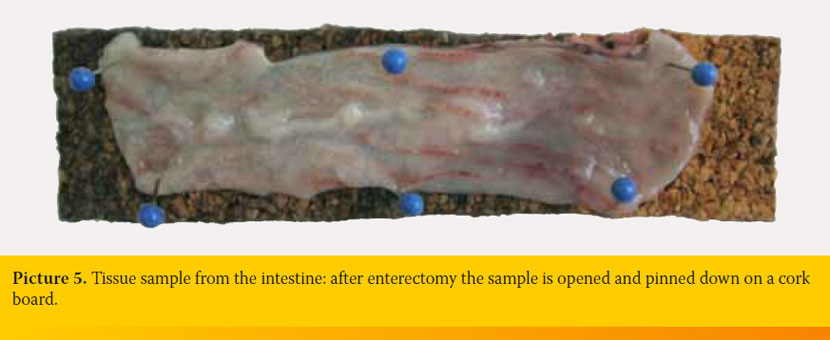
Samples from the intestine include biopsies obtained endoscopically or parts of the intestine excised during enterotomy or enterectomy. Endoscopically obtained intestinal biopsy samples, which have usually the size of a pin head, should be placed without delay in the fixative wrapped with filter paper or within biopsy cassettes. Two ways of handling are recommended for parts of intestine removed by enterectomy: 1) The excised part of the intestine is rinsed with saline which is carefully injected into the lumen using a syringe without needle. Following, the intestine is filled with formalin and placed for fixation. This way, collapse of the intestinal wall and damage of villi are avoided. The intestine can be sectioned longitudinally or transversely after the fixation. 2) The excised part of the intestine is incised along the mesenteric side. Following, it is rinsed thoroughly to remove the intestinal content and it is pinned down on a cork board with the mucosa facing upwards (Picture 5). Then it is placed in the fixative. This way, the intestine remains flat. Otherwise, contraction of the muscle layer would lead to intestinal folding.
Muscles
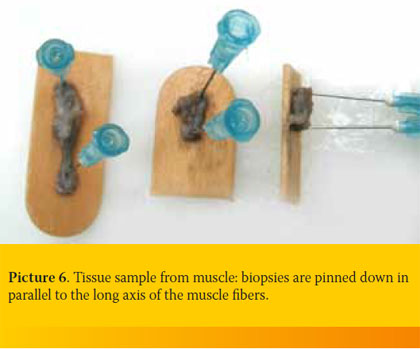 It is imperative that muscle biopsies are pinned down on a cork board or tongue depressor, otherwise muscle contractions will mask any existing lesions. Pinning down must be performed along the longitudinal axis of the muscle fibers (Picture 6). Alternatively, the tissue, before being placed in formalin, must be placed in normal saline for 15-30 minutes, so that the glycogen reserves are reduced.
It is imperative that muscle biopsies are pinned down on a cork board or tongue depressor, otherwise muscle contractions will mask any existing lesions. Pinning down must be performed along the longitudinal axis of the muscle fibers (Picture 6). Alternatively, the tissue, before being placed in formalin, must be placed in normal saline for 15-30 minutes, so that the glycogen reserves are reduced.
Testis
When histological examination of testes is performed for research purposes or when sperm cells structure must be evaluated (e.g. in azospermia/oligospermia) the fixative of choice is Bouin’s solution.
Bouin’s solution
Saturated aqueous picric acid solution 75ml
Formol (37-40% formaldehyde) 25ml
Glacial acetic acid 5ml
Bouin’s solution penetrates rapidly and does not cause hardening of the tissue.
> Handling of tissue and organs of dead animals
When organs and tissues intended for histopathological evaluation have been collected from deceased animals, they are handled the same way as previously described. The following describes the proper handling of brain samples.
Brain
Post-mortem the nervous tissue exhibits very soon autolytic changes, which cannot be easily distinguished from early degenerative lesions. To obtain best possible results, the brain should be placed in the fixative immediately after its removal from the scull. When there is a history of brain lesions, removal of the brain from the scull should precede any examination of other organs. In such cases formalin saline solution is the fixative of choice because it prevents hardening of the tissue externally, increasing the penetrance of formalin. After 24 hours the formalin solution is discarded, the brain is transversely dissected into 1cm thick slices and placed in fresh formalin for full fixation.
10% Formalin saline solution
Formol (37-40% Formaldehyde) 100 ml
Sodium chloride (NaCl) 90 gr
Tap water 900ml
> Referral form for histopathological examination
The form must contain:
- The animal signalment (age, gender, breed).
- The animal history (besides clinical signs, previous treatment should be indicated, because it may affect the histological features).
- Clinical or differential diagnosis.
- Other tests results (e.g. radiological, ultrasonographic evaluation and particularly results from cytological or previous histological examinations). This information contributes to accurate diagnosis and protection of the animal‘ s health.
- The way of sampling (total or partial resection, biopsy, aspiration etc.) and whether the whole tissue that has been removed is being shipped or only a part of it. It should be noted whether the biopsy has been obtained through endoscopy or if diathermy has been used, so that any tissue damage caused by manipulations will not be misinterprinted.
- Date of obtaining/fixation of the histological samples.
 > Shipment
> Shipment
- Sample containers with formalin should be placed in a box containing enough absorbent material (e.g. paper) in order to absorb potential leakage. The same applies when glutaraldehyde or paraforamldehyde solutions are used. If tissues have already been fixed for 24-48 hours prior to their shipment (with a minimum of fixative to tissue ratio 10:1), they can then be placed in a smaller container with less amount of fixative to facilitate their transportation and avoid any spillage. The samples are transported at ambient temperature.
- Frozen tissues are placed in a pre-cooled plastic container. The packaged specimen is shipped in a styrofoam box containing dry ice. The shipping container must be labeled with a “dry ice” indication.
- Most delivery companies are willing to undertake shipment of the samples. Tissues in formalin are not infective and dangerous for public health. In some cases, a declaration stating the above should accompany the transported biological material.
> Suggested reading
1. Kamstock DA, Ehrhart EJ, Getzy DM, Bacon NJ, Rassnick KM, Moroff SD, Liu SM, Straw RC, McKnight CA, Amorim RL, Bienzle D, Cassali GD, Cullen JM, Dennis MM, Esplin DG, Foster RA, Goldschmidt MH, Gruber AD, Hellmén E, Howerth EW, Labelle P, Lenz SD, Lipscomb TP, Locke E, McGill LD, Miller MA, Mouser PJ, O’Toole D, Pool RR, Powers BE, Ramos-Vara JA, Roccabianca P, Ross AD, Sailasuta A, Sarli G, Scase TJ, Schulman FY, Shoieb AM, Singh K, Sledge D, Smedley RC, Smith KC, Spangler WL, Steficek B, Stromberg PC, Valli VE, Yager J, Kiupel M; American College of Veterinary Pathologists’ Oncology Committee. Recommended guidelines for submission, trimming, margin evaluation, and reporting of tumor biopsy specimens in veterinary surgical pathology. Vet Pathol 2011, 48: 19-31.
2. Bancroft J, Gamble M. Theory and Practice of Histological Techniques. 5th edn. Churchill Livingstone.



