> Abstract
Prospective study of 19 cases
Canine chronic hepatitis (CH) is a group of liver diseases, the causes of which are different and little known, with similar clinical presentation and laboratory findings but with differences in the histological findings, prognosis and treatment.
The current prospective study concerns 19 cases of CH that were admitted, examined, diagnosed, treated and followed up at the Companion Animal Clinic, School of Veterinary Medicine, A.U.Th. between October 2012 and October 2014. During that period, 35 animals were admitted with clinical symptoms suggestive of liver disease. However, only 19 animals with histologically confirmed CH were included in the study.
The majority of dogs were of mixed-breed with a mean age of 6.5 years. The most common symptoms included depression, anorexia and vomiting. Though observed in certain cases, disorders in the blood count were uncommon. The main laboratory finding was that of increased liver enzyme activity: alanine aminotransferase (ALT) and alkaline phosphatase (ALP). Histopathology revealed a varying degree of inflammation, mostly involving lymphocytes and plasma cells in every case, fibrosis in several cases and apoptosis and/or necrosis and regeneration in few cases. Finally, one case was characterized as copper-associated CH.
> Introduction
Canine chronic hepatitis (CH) is a group of parenchymal liver diseases, the causes of which are different and largely unknown, with similar clinical presentation and laboratory findings. However, there are differences concerning histological findings (varying degree of hepatocellular necrosis and/or apoptosis, inflammatory cell infiltration, regeneration and fibrosis), prognosis and treatment.1 It is obvious that the disease cannot always be adequately characterized in terms of its aetiology, either clinically or histopathologically. Various terms have been assigned to the disease that imply the common clinical characteristics but not the histological findings. Such terms include chronic active hepatitis, chronic persistent hepatitis, lobular dissecting hepatitis, hepatitis with acidophilic bodies, non-specific reactive hepatitis, eosinophilic hepatitis e.t.c.2 In order to overcome this discrepancy, it was decided that the term chronic hepatitis should be used according to the severity of the inflammation or/ and fibrosis of the liver parenchyma, the stage, and the cause (if known) of the disease.3
The aetiology of CH is wide, involving infectious agents, drugs or toxins causing idiosyncratic and/or dose-dependent reactions and immunemediated mechanisms.4 When known causes of CH are excluded, the disease is characterized as idiopathic. Finally, copper-associated hepatitis of specific dog breeds is considered to be another type of CH. Bedlington Terriers are familiarly affected by the COMMD1 gene being genetically mutated, thereby altering the structure of the protein that is responsible for hepatic copper excretion.5-7 As copper accumulates in the liver parenchyma, the final result is its functional and morphological disorder, inflammation and necrosis. Other dog breeds (Doberman Pinscher, Labrador Retriever, West Highland White Terrier, Skye Terrier, Dalmatian, Cocker Spaniel, English Springer Spaniel) are also affected by this particular type of CH, though it has not yet been clarified whether genetic mutation is involved.8-24
The history and clinical presentation of dogs with CH vary, without being suggestive of the disease or the underlying cause. Symptoms are usually non-specific, with periods of remission and exacerbation such as anorexia, depression, jaundice, hepatodynia, vomiting, diarrhoea, weight loss, polyuria-polidipsia. Ascites and symptoms of hepatic encephalopathy suggest liver failure due to end-stage CH or acute-onchronic liver disease. It should be mentioned that many cases of CH might appear clinically healthy during clinical examination (periods of remission). In such cases, it is important to check liver enzyme activity in the blood serum (ALT, ALP, albumins, bile acids, glucose). 25
Diagnosis of CH is based on the history (breed, age, diet, drug administration before the onset of the disease, habitat, vaccination and previous history) and clinical and laboratory findings. Histological examination of biopsy specimens is essential for definitive diagnosis.2
In subclinical stages of the disease, the dogs show no symptoms other than increased liver enzyme activity.4 Increased serum activity of alanine aminotransferase (ALT) is strongly suggestive of hepatocellular damage in 75%- 95% of dogs with CH. 2,26,27 Enzyme levels can increase up to ten times above the upper limit of the reference range.27 In 85% of dogs with CH, the enzyme activity is commonly two to five times above the upper reference range. The activity of the enzyme aspartate transaminase (AST), is also frequently elevated. When chronic hepatitis is in remission or the disease is endstage (cirrhosis) and the volume of the remaining healthy parenchyma is small, liver enzyme activity may be normal,27 depending on the severity of the inflammation of the liver, degree of cellular necrosis and half-life of each enzyme. Other less common biochemical disorders include hyperbilirubinaemia, hypoalbuminaemia and hypogammaglobulinaemia, low urea nitrogen and hypoglycaemia (end-stage liver disease). 2,28 In such cases, measuring the pre- and postprandial total serum bile acid concentrations could reveal liver disfunction.25 Serum cholesterol concentration can be either high, normal or low.2
Haematologic abnormalities may include mild non-regenerative anaemia of chronic disease, regenerative anaemia due to blood loss from coagulopathy, or gastrointestinal ulcers.4 Haemolytic anaemia in copper-associated hepatitis has only been reported in Bedlington Terriers.4 Prolonged prothrombin time (PT) and partial thromboplastin time (PTT) suggest severe liver disease. 2,18,29
Survey abdominal radiographs and ultrasonography can reveal useful information concerning the size of the liver (hypoechogenic, small-sized liver in animals with cirrhosis), the presence of acquired portosystemic shunts due to chronic portal vein hypertension, ascites, thickening of the bile ducts, choleliths, and disturbance of the bile flow. 4,25,30
Definite diagnosis is confirmed only after liver biopsy and histopathological examination of the specimen.
The purpose of the present prospective study was to investigate the epidemiological data and the clinical and laboratory findings of canine CH. To the authors’ knowledge, there are no previous reports concerning the disease in Greece.
> Materials and Methods
The current prospective study concerns 19 cases of CH that were admitted, examined, diagnosed, treated and followed up at the Companion Animal Clinic, School of Veterinary Medicine, A.U.Th. between October 2012 and October 2014.
All cases were selected among 35 dogs that were presented to the clinic during the aforementioned period of time exhibiting symptoms compatible with liver disease that was later confirmed by histopathology. The 19 cases which were finally included exhibited clinical and laboratory findings compatible with those of chronic liver disease and histopathological features of CH such as necrosis, chronic inflammation and regeneration. Specifically, the only animals enrolled in the study were those whose biopsies revealed CH according to WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases, WSAVA Liver Standardization Group, 2006.3 Animals with different histopathological diagnosis (e.g. neoplasm, fatty liver, vascular anomalies, etc.) were not included in the study.
For each animal, the sex, age, breed, body weight and a 5-scale body condition score and presenting complaint were recorded.
Upon clinical examination, the body temperature, heart rate, possible dehydration, mucus membrane and skin examination for signs of jaundice, palpation of the abdomen and particularly the liver, heart and thorax auscultation and rectal examination were also recorded.
Blood analysis was performed at the Diagnostic Laboratory, School of Veterinary Medicine, A.U.Th. The values of blood parameters mentioned in the current study refer to those detected at first admission to the clinic.
The blood counts were analyzed using the haematology analyzer ADVIA 120, Healthcare Diagnostics, Michigan, USA. The parameters measured were haematocrit (HCT), haemoglobin (Hb), and total white blood cells (WBC), while partial white cell counts consisted of neutrophils, lymphocytes, monocytes, eosinophils, basophils and platelets (PLT). The concentration of total solids was determined by a handheld refractometer (American Optical Leica TS Meter, American Optical Co, Massachusetts, USA).
Serum biochemical parameters were determined using a chemistry analyzer Vital Scientific N.V. Flexor E, AC Dieren, Netherlands. The concentration of potassium and sodium were determined by the analyzer Roche mod. 9180 Electrolyte Analyzer, Mannheim, Germany and that of the bile acids by IDEXX Laboratories Vet Test Chemistry Analyser 8008, Snap Reader Series II, Maine, USA.
The concentration of the blood urea nitrogen (BUN), creatinine (Crea), albumin (Alb), glucose (Gluc), total bilirubin (TBil), direct bilirubin (DBil), cholesterol (Chol), triglycerides (Trig), potassium (K), sodium (Na), ammonia (NH3) and bile acids (BA) as well as the activity of alkaline phosphatase (ALP), alanine aminotransferase (ALT) and γ-glutamyl transferase (γ-GT) were recorded. Bile acid concentration (a 12-hour fasting preprandial and a 2-hour postprandial sample) was recorded only in 9/19 cases due to the lack of strong evidence supporting liver disease or financial restriction of the owner.
Urine was collected by cystocentisis or ‘free catch’. The specific gravity was determined with a handheld refractometer. A urine test strip was used to determine the pH and to detect the presence of glucose, blood, proteins, haemoglobin, bilirubin and urobilinogen. The urine was centrifuged (1500 rpm/3 min) and the sediment was microscopically examined for the presence of red blood cells, white blood cells, bacteria, crystals and casts.
Imaging results were also recorded, with the emphasis on those referring to the liver. The examination was performed at the Diagnostic Imaging Unit, School of Veterinary Medicine, A.U.Th. with a Polydoros 80, Siemens, Munich, Germany x-ray generator. The results of the ultrasonographic examination were also recorded. The examination was performed with an Apogee Advanced Technologies, Bothell WA, USA or an Acuson X300, Siemens, Munich, Germany ultrasound system.
Biopsy specimens were obtained percutaneously in all cases under ultrasonographic guidance using a semi-automatic True-cut needle (EASYRAM, Semiautomatic guillotine needle for soft-tissue biopsy, 14G X 130mm, RI.MOS., Mirandola, Italy). All dogs were kept under shortterm general anaesthesia (dexmedetomidine, propofol). The specimens where then fixed in 10% buffered formalin and transferred to the neutral of Pathology, School of Veterinary Medicine, A.U.Th.
After fixation, the specimens were embedded in paraffin and cut into 4-6 μm thick sections. The sections were stained with haematoxylin and eosin to detect liver lesions and rhodanine for copper accumulation.
> Results
Nineteen out of 35 cases met the inclusion criteria for canine CH.
Seven out of the 19 dogs with CH (36.8%) were of mixed breed, 2/19 (10.5%) were Cocker Spaniels, 2/19 (10.5%) Doberman Pinchers, 2/19 (10.5%) German Shepherds and one dog belonged to each of the following breeds (5.2%) West Highland White Terrier, Yorkshire Terrier, Pekingese, Jack Russell Terrier, Shih-Tzu and a Rottweiler. Moreover, 11/19 (57.8%) were male, while the rest 8/19 (42.1%) were female. Three out of 11 male dogs (27.2%) were castrated but none of the females was spayed (Diagram 1).
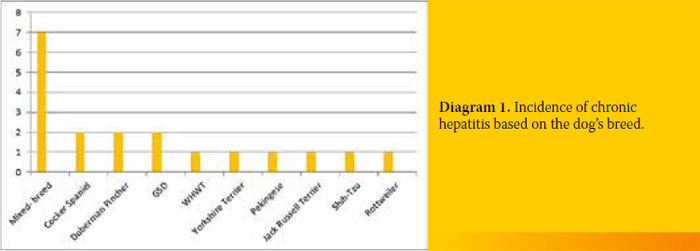
The dogs were aged between 2 to 15 years (mean age 6.5 years). Their body weight ranged from 2.95 kg to 39.7 kg (mean weight 14.95kg).
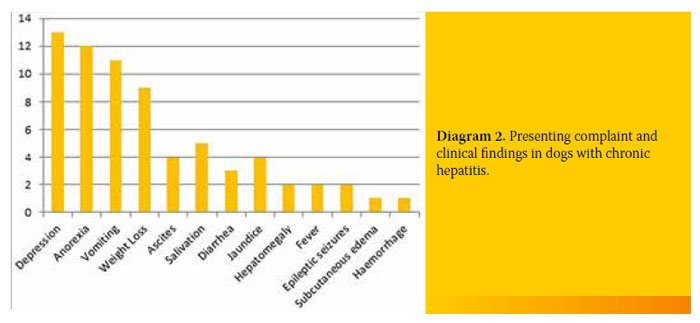
Most of the dogs with CH were admitted to the clinic due to depression (13/19, 68.4%). Twelve out of 19 dogs (63.1%) exhibited anorexia and 11/19 (57.9%) vomiting. Other symptoms included weight loss (9/19, 47.4%), ascites (4/19, 21.1%), jaundice (4/19, 21.1%), diarrhoea (3/19, 15.8%), seizures (2/19, 10.5%), subcutaneous oedema (1/19, 5.3%) and bleeding disorders (1/19, 5.3%). Reasons for admission and the incidence in the 19 dogs with CH are shown in Diagram 2.
The most important findings of complete blood count were thrombocytopenia (4/19, 21.1%) and leukocytosis (4/19, 21.1%). Three out of 19 dogs had anaemia (15.8%).
Regarding biochemical results, ALT was elevated in 17/19 (89.5%), ALP was elevated in 17/19 (89.5%), and γ-GT was 8/8 (100%) in all study dogs measured. Finally, the serum concentration of bile acids (12-hour fasting preprandial and/or 2-hour postprandial sample) was increased in 9/9 measured.
The urinalysis results of all the animals were unremarkable.
Abdominal radiographs revealed a small-sized liver in 3/19 (15.8%) dogs but no animal exhibited cholelithiasis or biliary “sludge”.
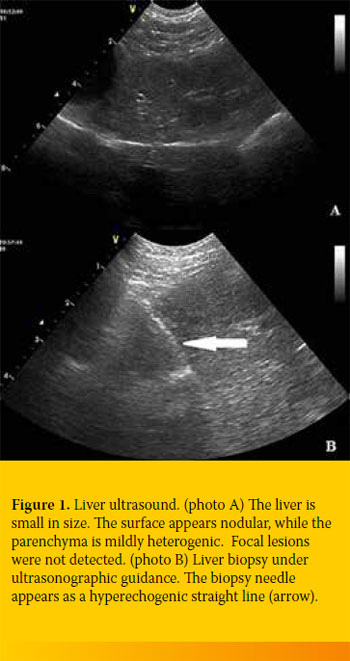
The liver was ultrasonographically checked at the time of biopsy. Echogenicity differed among cases, but in general it appeared increased at varying degrees (Figure 1). The biliary system and the gallbladder reflected no specific pathology in our cases.
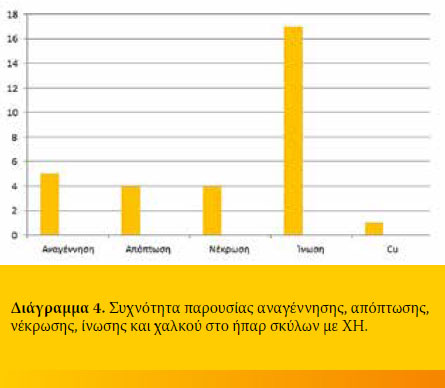
A biopsy specimen was taken from each dog with CH. Histology revealed regeneration in 5/19 (26.3%), apoptosis in 4/19 (21.1%) and necrosis in
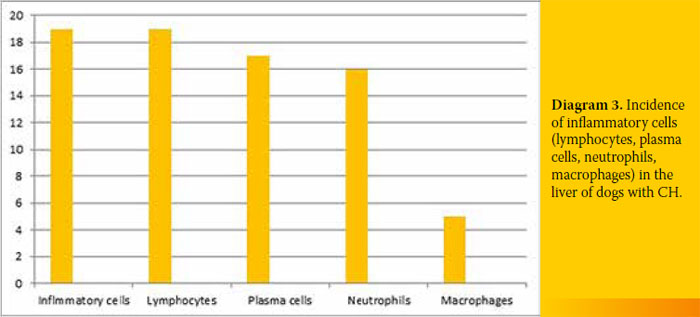
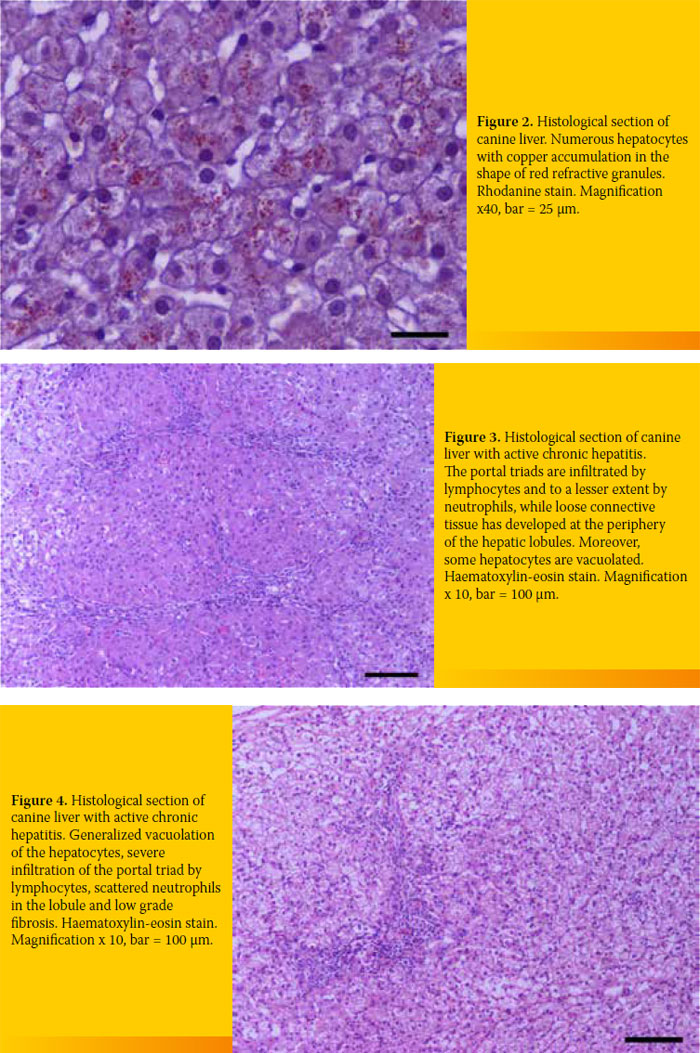
4/19 (21.1%). All specimens were infiltrated with inflammatory cells 19/19 (100%), the amount of which differed among cases. Finally, extensive copper accumulation was found in 1/19 dogs (Diagram 3 and 4, Figure 2-4).
After histological confirmation, all animals received aetiological and supportive care. Aetiological care included prednisolone in immunosuppressive dosage and supportive care involved S-Adenosyl-L-methionine (SAMe) in combination with ursodeoxycholic acid and a commercial clinical diet (Hepatic, Royal Canin).
To the authors’ knowledge, one animal died due to liver failure while the others remain alive and in good clinical condition. The animals were monitored for a minimum period of six months to a maximum of 20 months after their first admission to clinic.
> Discussion
The purpose of the present study was to investigate the epidemiological data as well as the clinical and laboratory findings of dogs with CH. The main reason for conducting the study was the lack of clinical data concerning the disease in Greece.
The incidence of CH has risen in recent years (unpublished data, Companion Animal Clinic, Unit of Internal Medicine, School of Veterinary Medicine, A.U.Th). Thirty-five dogs with histopathologically confirmed liver disease were admitted between October 2012 and October 2014, nineteen of whom presented CH and were finally enrolled in this study. However, this number does not reflect the real prevalence as a substantial number of owners refused to consent to further investigation or liver biopsy.
The limited caseload of animals with CH did not allow statistical analysis, but was sufficient for drawing useful conclusions.
Chronic hepatitis is a disease commonly encountered by clinicians who are frequently obliged to treat the animal without a definite diagnosis, mainly because of financial reasons or hesitation concerning possible and unsustainable side-effects for the dog. More often than not, the clinician is forced to provide treatment based on the clinical and laboratory findings, which is why the results of the current study are important.
The results presented herein show that CH affects mostly middle-aged, mixed-breed dogs. This finding conflicts with the results of previous studies which report a predisposition among Bedlington terriers, West Highland White Terriers, Skye Terriers, Doberman Pinschers, Dalmatians, Labrador Retrievers and Cocker Spaniels.5-26 In such breeds, CH is related to copper accumulation. In the present study, only one case involving a West Highland White Terrier was recorded with copper accumulation in the liver as confirmed by rhodamine staining. Chronic hepatitis was not correlated with copper accumulation in the two Cocker Spaniels and the two Doberman Pinscher cases that were enrolled in the study. The selected method of biopsy, percutaneous over open biopsy, did not allow for quantitative assessment of the liver’s copper inside the liver parenchyma. It is possible that the presumably large number of mixed breed dogs enrolled in our study is because a considerable number of such dogs are admitted to the Companion Animal Clinic. The results of our study indicate that it is not just the pure-bred dogs that are susceptible to CH, implying that hereditary reasons are not the only responsible causes of the disease.5-24 According to Poldervaart et al.26 and Fuentealba et al.,31 the disease may affect any breed, sex and/or age.
The most common reason for admission was depression, confirmed upon clinical examination (68.4% of the dogs). The results of the present study are in accordance with other studies.2,4 In contrast, one study conducted at the university of Utrecht reported a lower incidence (18%).4 Depression is the result of many co-existing factors which depend on the stage of CH. Ascites, found in 4/19 cases, was attributed to changes in the metabolic rhythm and decrease of liver biotransformation ability. 1,2,4
Vomiting is a common finding in dogs with CH. The percentage of the animals exhibiting vomiting in our study (57.8%) is higher than that reported in other studies (40%-50%).2,25 Vomiting is almost consistently recorded in all types of liver disease; hence, the clinician should investigate further after excluding gastric disorders. This clinical symptom is the result of chronic liver inflammation and/or the organ’s disability to inactivate toxins which impact directly on the emetic chemoreceptor trigger zone. 1,4
Anorexia and weight loss are reported in 63.1% and 57.8% of the dogs, respectively. Even though anorexia is generally recognized as a common symptom in dogs with CH, other studies report a lower incidence of up to 50%. As far as anorexia is concerned, the incidence in our study is also greater compared to that reported in other studies (28%-40%).26,28 Metabolic disorders ascribed to liver disease, vomiting, inflammation of the liver parenchyma and their impact explain the aforementioned symptoms.
Four out of 19 cases (21%) developed ascites which was successfully cured with aetiological and supportive treatment, a finding that is not commonly reported in the literature.2,33 Ascites in such cases is the result of acute-on-chronic liver disease. In other words, ascites is the result of transient liver failure without permanent morphological changes in the liver parenchyma (cirrhosis, neoplasm). To support this aspect, it should be mentioned that serum albumin concentration was not low and that ascites resolved after treatment.
According to the history and clinical examination, 5/19 cases presented salivation. According to the literature, salivation is rarely encountered.
The remainder of the clinical findings of this study pertain to a limited number of cases and are not discussed further.
ALT activity was increased two-fold up to over four-fold in 17/19 cases. This increase implies a serious and massive inflammation and/or hepatocellular necrosis as a result of the causative agent of CH. In 2/19 cases, the activity of ALT was within normal limits. However, the clinical examination of those animals strongly suggested liver disease; bile acid concentrations were also measured to detect the liver functional ability and revealed liver failure. Normal activity of ALT can be attributed to the nature of the disease (periods of active and/or silent) and the short half-life of the enzyme.31,32
ALP activity increased two-fold up to over fourfold in 17/19 cases. Clinical examination did not identify any possible extrahepatic sources of ALP increase (young, lactating or pregnant animals, animals with mammary or bone cancer, pyometra). The increased activity of ALP coexists with that of ALT in the majority of chronic diseases of the liver, reflecting the inability of the inflamed liver cells to recycle the bile acids and the cholestasis resulting from the morphological changes in the biliary system (inflammatory cells infiltrate the parenchyma surrounding the bile ducts).
Increased serum bilirubin can be attributed to the aforementioned reasons. Furthermore, jaundice develops in just 30%-40% of liver diseases .
Four cases exhibited hypoalbuminaemia and ascites simultaneously; the pathophysiology of the former has already been mentioned.
Serum albumin concentration in the above cases was not lower than 2 mg/ 100ml; this finding is in accordance with the aetiopathogenesis of acuteon- active liver failure. Our observations in cases of chronic liver failure due to cirrhosis identified more severe hypoalbuminaemia (<2mg/ 100ml) than that noted in the current study.
One dog presented epilepsy concurrently with ascites which was attributed to hepatic encephalopathy confirmed by hyperammonemia.
Definite diagnosis of CH was based on histopathological diagnosis.4 Infiltration of inflammatory cells was found in all cases (lymphocytes, plasma cells, and white blood cells to a lesser extent). Seventeen out of 19 cases exhibited varying degrees of fibrosis (including bridging fibrosis) and necrosis and/or apoptosis and regeneration. These histological findings can differ among cases and should be taken into account for diagnosis (Figures 2-4).
The clinician should combine the histological, clinical and laboratory findings and decide the extent to which the disease is ‘active’ so that he can prescribe the proper treatment and give a prognosis.
The degree of the hepatic disorder may be estimated by the intensity of inflammation and the extent of apoptosis and/or necrosis. On the other hand, determining the kind and the extent of cirrhosis can determine the prognosis. The presence of regenerative nodules in the liver parenchyma usually implies a better prognosis. As far as copper-associated hepatitis is concerned, the histopathological examination did not reveal regeneration, apoptosis and/or necrosis apart from mild inflammatory cell infiltration and a case of extended copper accumulation as shown after staining with rhodanine. This particular case involved a West Highland White Terrier, a breed genetically predisposed to copper-associated hepatitis (Figure 2).16
Animals with symptoms and laboratory findings suggestive of liver disease should undergo a liver biopsy, the results of which will determine the type of treatment and prognosis
> References
1. Ράλλης ΤΣ. Νοσήματα του ήπατος. Από: Γαστρεντερολογία του σκύλου και της γάτας. Ράλλης ΤΣ. 2η εκδ. University studio press: Θεσσαλονίκη, 2006, σελ. 231- 233.
2. Center SA. Chronic Hepatitis, Cirrhosis, Breed- Specific Hepatopathies, Copper Storage Hepatopathy, Suppurative Hepatitis, Granulomatous Hepatitis, and Idiopathic Hepatic Fibrosis. In: Strombeck’s Small Animal Gastroenterology. Guilford WG, Center SA, Strombeck DR. 3rd ed. WB. Saunders: Philadelphia, 1996, pp. 705- 765.
3. Van den Ingh T, van Winkle T, Cullen JM, Charles JA, Desmet VJ. Morphological classification of parenchymal disorders of the canine and feline liver, 2, Hepatocellular death, hepatitis and cirrhosis. In: WSAVA Standards for Clinical and Histological Diagnosis of Canine. WSAVA Liver Standardization Group. WSAVA Liver Standardization Group. WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases. Elsevier: Toronto, 2006, pp. 85-100.
4. Washabau RJ. Liver. In: Canine and Feline Gastroenterology. Washabau RJ, Day M. WB. Saunders: Philadelphia, 2013, pp. 849-957.
5. Twedt DC, Sternlieb I, Gilbertson SR. Clinical, morphologic, and chemical studies on cooper toxicosis of Bedlington Terriers. J Am Vet Med Assoc 1979, 175: 269-275.
6. Hultgren BD, Stevens JB, Hardy RM. Inherited chronic progressive hepatic degeneration in Bedlington Terriers with increased copper concentrations: Clinical and pathologic observations and comparison with other copper associated liver diseases. Am J Vet Res 1986, 47: 365-377.
7. Van de Sluis B, Rothuizen J, Pearson PL, van Oost BA, Wijmenga C. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum Mol Genet 2002, 11: 165-173.
8. Mandigers PJ, van den Ingh TS, Bode P, Teske E, Rothuizen J. Association between liver copper concentration and subclinical hepatitis in Doberman Pinschers. J Vet Intern Med 2004, 18: 647-650.
9. Johnson GF, Zawie DA, Gilbertson SR, Sternlieb I. Chronic active hepatitis in Doberman pinschers. J Am Vet Med Assoc 1982, 180: 1438-1442.
10. Speeti M, Stahls A, Meri S, Westermarck E. Upregulation of major histocompatibility complex class II antigens in hepatocytes in Doberman hepatitis. Vet Immunol and Immunopathol 2003, 96: 1-12.
11. Haywood S, Rutgers HC, Christian MK. Hepatitis and copper accumulation in Skye terriers. Vet Pathol 1988, 25: 408-414.
12. Hoffmann G, van den Ingh TS, Bode P, Rothuizen J. Copper-associated chronic hepatitis in Labrador retrievers. J Vet Intern Med 2006, 20: 856-861.
13. Shih JL, Keating JH, Freeman LM, Webster CR. Chronic hepatitis in Labrador Retrievers: Clinical presentation and prognostic factors. J Vet Intern Med 21: 2007, 33-39.
14. Smedley R, Mullaney T, Rumbeiha W. Copperassociated hepatitis in Labrador Retrievers. Vet Pathol 2009, 46: 484-490.
15. Webb CB, Twedt DC, Meyer DJ. Copper-associated liver disease in Dalmatians: A review of 10 dogs (1998– 2001). J Vet Intern Med 2002,
16: 665-668. 16. Thornburg LP, Rottinghaus G, Dennis G, Crawford S (1996): The relationship between hepatic copper content and morphologic changes in the liver of West Highland White Terriers. Vet Pathol 1996, 33: 656-661.
17. Mizooku H, Kagawa Y, Matsuda K, Okamoto M, Taniyama H. Histological and immunohistochemical evaluation of lobular dissecting hepatitis in American Cocker Spaniel dogs. J Vet Med Sci 2013, 75(5): 597-603.
18. Watson P, Skancke E, Farstad W, Sampson J, Bloomfield L, Scott L. Hepatitis in English Springer Spaniels in the UK and Norway. Vet Rec 2006, 158: 311.
19. Jensen AL, Nielsen OL. Chronic hepatitis in three young Standard Poodles. Zentralbl Veterinarmed 1991, A 38: 194-197.
20. Andersson M, Sevelius E. Breed, sex and age distribution in dogs with chronic liver disease: A demographic study. J Small Anim Pract 1991, 32: 1-5.
21. Rolfe DS, Twedt DC. Copper- associated hepatopathies in dogs. Vet Clin North Am Small Anom Pract 1995, 25: 399-417.
22. Watson PJ. Chronic hepatitis in dogs: a review of current understanding of the aetiology, progression and treatment. Vet J 2004, 167: 228-241.
23. Thornburg LP, Rottinghaus G, McGowan M, Kupka K, Crawford S, Forbes S. Hepatic copper concentrations in purebred and mixed-breed dogs. Vet Pathol 1990, 27: 81-88.
24. Bexfield NH, Buxton RJ, Vicek TJ, Day MJ, Bailey SM, Haugland SP, Morrison LR, Else RW, Constantino-Casas F, Watson PJ. Breed, age and gender distribution of dogs with chronic hepatitis in the United Kingdom. Vet J 2012, 193: 124-128.
25. Favier RP. Idiopathic hepatitis and cirrhosis in dogs. Vet Clin North Am Small Anim Pract 2009, 39: 481-488.
26. Poldervaart JH, Favier RP, Penning LC, van den Ingh TS, Rothuizen J. Primary hepatitis in dogs: a retrospective review (2002-2006). J Vet Intern Med 2009, 23: 72-80.
27. Center S. Interpretation of liver enzymes. Vet Clin Am Small Anim Pract 2007, 37: 297-333.
28. Sevelius E. Diagnosis and prognosis of chronic hepatitis and cirrhosis in dogs. J Small Anim Pract 1995, 36: 521-528.
29. Prins M, Schellens CJ, van Leeuwen MW, Rothuizen J, Teske E. (2010): Coagulation disorders in dogs with hepatic disease. Vet J 2010, 85(2): 163-168
30. Gaschen L. Update on Hepatobiliary Imaging. Vet Clin North Am Small Anim Pract 2009, 39: 439-467.
31. Fuentealba C, Guest S, Haywood S, Horney B. Chronic hepatitis: A retrospective study in 34 dogs. Can Vet J 1997, 38: 365-373.
32. Fleisher GA, Wakim KG. The fate of enzymes in body fluids–an experimental study. Disappearance rate of glutamic-pyruvic transaminase under various conditions. J Lab Clin Med 1963, 61: 76.
33. Boyd JW. The mechanisms relating to increases in plasma enzymes and isoenzymes in diseases of animals. Vet Clin Pathol 1983, 12: 9.



