> ABSTRACT
Spirocerca lupi infection is an uncommon cause of pyothorax in the dog. In the present report, two cases of canine spirocercosis-associated pyothorax are described. Both cases demonstrated historical or clinical evidence of esophageal dysphagia, manifested as odynophagia and/or regurgitation, and one showed clinical findings suggestive of pleural effusion such as weakness, depression and muffled heart sounds. Thoracic radiography in the first case revealed pleural effusion and soft tissue opacity located at the caudodorsal aspect of the mediastinum, while S. lupi ova were found in fecal examination of both dogs. The first dog was euthanized at his owner’s request, while the second died suddenly during hospitalization. The definitive association between spirocercosis and pyothorax was established post mortem. These cases emphasize the importance of considering esophageal spirocercosis as a cause of canine pyothorax in highly endemic areas.
> INTRODUCTION
Pyothorax is defined as the presence of septic exudate in the pleural cavity.1 Although in the majority of cases no source of infection can be identified, pyothorax may occur as a result of penetrating thoracic wounds, migrating foreign bodies, thoracic neoplasia, pulmonary infection, hematogenous spread of infectious agents and various medical procedures (e.g. esophageal rupture during esophagoscopy).2 Spirocercosis is caused by the nematode Spirocerca lupi and is found worldwide, with the majority of recent reports originating from Israel, Greece and South Africa.3-6 The parasite affects mostly canines, although it has been recognized in a range of carnivorous species. The adult worms are commonly found embedded in one to several nodules in the host’s caudal esophagus, depositing larvated eggs in the lumen, and passing out in the feces. The pathology of spirocercosis results mainly from larval migration, presence of adult worms in nodules in the esophageal wall and the transformation of nodules into sarcomas. Major clinical signs include esophageal dysphagia manifested with excessive salivation, esophagodynia and regurgitations, weight loss and vomiting.4,5,7 Aortic lesions are usually asymptomatic, unless rupture occurs, resulting in hemothorax and sudden death. Although spirocercosis is rarely considered to be involved in canine pyothorax, a few cases of concurrent pyothorax and esophageal spirocercosis have been reported in the literature. However, a cause-and-effect relationship between the two entities has not always been straightforward. 1,5,8-13 This report describes two canine cases of S. lupi-associated pyothorax, focusing on their clinical, clinicopathological and pathological features along with the pathogenesis of the S.lupiassociated pyothorax.
> CASE REPORTS
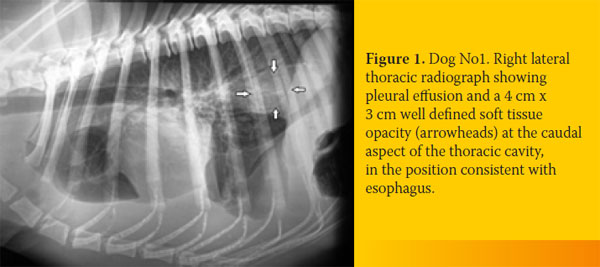
Case No. 1
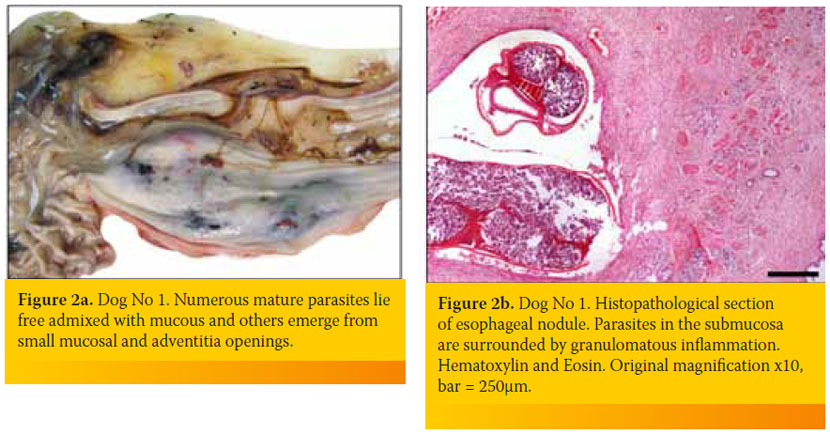
An 8-year-old intact male, German Shepherd dog was admitted because of weakness of two days duration. The dog lived outdoors and was fully vaccinated. Its owner reported a history of progressive weight loss in the context of a normal appetite and an episode of regurgitation four days earlier. Physical examination identified depression, poor body condition, tachycardia with muffled heart sounds, weakness, dehydration and watery stools. A complete blood count revealed a moderate non-regenerative (reticulocyte production index: < 1; lower reference limit: < 2) anemia (hematocrit: 26.3%; reference range, 37-55%), leucocytosis (58,400/μl; reference range: 6,000-17,000/μl) with neutrophilia (48,760/μl; reference range: 3,000-11,000/μl) and a regenerative left shift (bands 2,500/μl, reference range: 0-300/ μl) and monocytosis (5,540/μl; reference range: < 1,350 /μl). Serum biochemistry abnormalities included hypoalbuminemia (2 g/dL; reference range, 2.9-4 g/dL), hyperbilirubinemia (1.6 mg/dL; reference range, 0.2-0.6 mg/dL), hypoglycemia (42 mg/dl; reference range, 65-118 mg/dl) and increased alkaline phosphatase activity (1,027 U/L; reference range, 32-149 U/L). A sample of orangecolored urine was obtained by cystocentesis and urinalysis revealed bilirubinuria, while microscopic examination of sediment identified bacteriuria. Embryonated S. lupi ova were found in the fecal examination (Teleman’s sedimentation technique). Lateral, dorsoventral and ventrodorsal radiographs demonstrated pleural effusion and soft tissue opacity located at the caudodorsal aspect of the mediastinum. The mass was in the position consistent with the esophagus, but the existence of a non-esophageal mediastinal mass could not be excluded (Figure 1). A sample of hemopurulent pleural fluid obtained by a butterfly-assisted thoracocentesis was examined cytologically; numerous degenerated neutrophils including several with phagocytized bacteria were noted, establishing the diagnosis of pyothorax. Due to the guarded-to-poor prognosis, the dog was euthanized at the owner’s request. At necropsy, approximately 750 ml of hemopurulent foul-smelling exudate filled the pleural space. A sizeable mass measuring 10cm x 5cm was observed at the caudal esophagus. Nematode parasites protruded from the adventitia of the mass and multiple free-living nematodes were found in the esophageal lumen or protruding from small openings of the mucosa (Figure 2a). On cut surface, the mass was firm, solid and reddish-to-grey in color, with numerous cavities of varying sizes containing necrotic/ purulent material and/or parasites. Occasional canal formations due to communications between adjacent cavities were noted. The small openings and cavities described above caused direct communications between the esophageal lumen and the thoracic cavity. Multiple nodules, smaller than 0.5 cm, were detected mainly on the wall of the thoracic aorta. Intimal roughening and small aneurysms of the aorta were also observed. Histopathological examination of the esophageal mass revealed the presence of parasites, granulomatous and, to a lesser extent, pyogranulomatous inflammation, as well as development of excess fibrous connective tissue (Figure 2b).
Case No. 2
A 3-year-old intact female mixed breed dog was presented because of tarry stools and a twoweek history of decreased appetite. The dog was housed outdoors, with seven other dogs and was fully vaccinated. The dog had a history of mild depression, abdominal pain and an episode of hematemesis two days earlier. Physical examination revealed melena, dehydration, depression and mucosal pallor. During a feeding trial with canned food, odynophagia and a regurgitation episode were also documented.
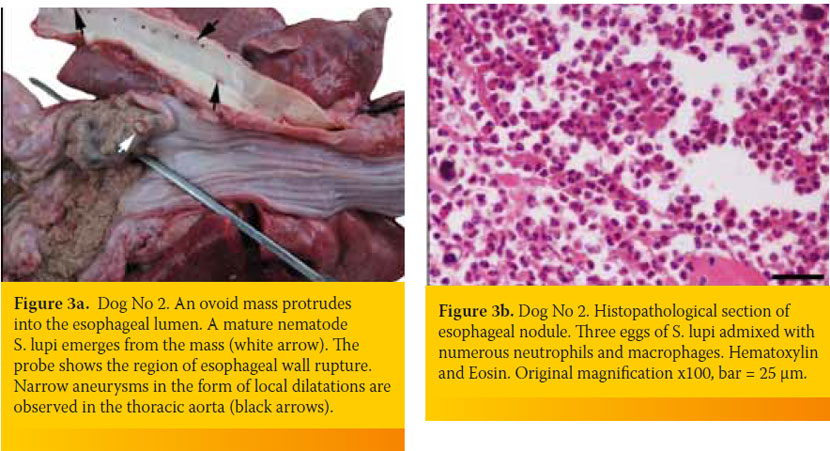
A complete blood count revealed a severe regenerative (reticulocyte counts: 363,000/ μl; reference range <130,000/ μl), microcytic (mean corpuscular volume: 58.5 fL; reference range: 61-77 fL) and hypochromic (mean corpuscular hemoglobin concentration: 27.4 g/dL; reference range: 31-36 g/dL) anemia (hematocrit: 13.7%; reference range, 37.1-55%) and leucocytosis (36,050/μl; reference range: 6,000-17,000/μl) with neutrophilia (28,040/μl; reference range: 3,900-8,000/μl). A comprehensive serum biochemistry profile was within normal ranges. At fecal examination (Teleman’s sedimentation method), S.lupi ova were found. Thoracic and abdominal survey radiographs were unrewarding. The dog was hospitalized and a unit of blood-typed and crossmatched whole blood was transfused; however, after a few hours the dog developed fever (39.6oC) and the next morning it suddenly died. Necropsy revealed the presence of approximately 300 ml of turbid, fibrino-purulent and hemorrhagic exudate in the pleural cavity, along with pulmonary atelectasis and visceral pleural thickening. In the caudal esophagus a severe dilatation and a sizable rupture (3 x 4cm) with an outflow of food conglomerate, retained by the pleura were witnessed. In the same area, a firm ovoid nodule measuring 2cm x 3cm was seen to arise from the esophageal wall and project into its lumen. The latter was located adjacent to the rupture and adult S. lupi worms protruded from the nodule’s surface through small openings (Figure 3a). Removal of a food bolus revealed that the edges of the rupture were hemorrhagic and uneven. In the aorta, endarterial coarseness as well as small and narrow aneurysms were seen. Histopathology of the esophageal nodule showed extensive pyogranulomatous inflammation and presence of mature parasites and eggs (Figure 3b). Furthermore, the wall of the distal esophagus displayed extensive ulceration and generalized inflammatory cell infiltration consisting mostly of neutrophils and macrophages. A similar but less intense inflammation was observed in sections taken from the aorta.
> DISCUSSION
In the present study, a causal relationship was established between esophageal spirocercosis and pyothorax, as several perforations on the parasitic mass (case 1) and a rupture of the esophageal wall in the close vicinity of the parasitic nodule (case 2) were documented post mortem, creating a communication between the esophageal lumen and the pleural cavity. Interestingly, of the 13 cases of S. lupi-associated pyothorax reported to date, an evidence-based association between the two entities was only reached in eight dogs based on either post mortem examination (six dogs) or contrast esophagography that demonstrated leakage of the contrast material into the pleural cavity (two dogs).10-13 Five additional cases with concurrent pyothorax and spirocercosis have also been described, and although their association was strongly suspected, a definitive confirmation was not established.5,11 Several theories concerning the pathogenesis of S. lupi-associated pyothorax have been proposed. Gross perforation of the nonneoplastic or neoplastic nodules, due to deep ulceration, necrosis or massive passage of adult worms, has been the most plausible explanation.11,14 Alternatively, microscopic perforations of the nodules by the aberrant migration of worms towards the adventitia rather than the mucosal surface of the esophagus may also cause pyothorax by spillage of eggs, pus and excretions of the worms into the thoracic cavity.15 In the latter case, no endoscopic or contrast radiographic evidence of perforation would be expected.16 Moreover, nodules located at the distal area of the esophagus, producing lower esophageal sphincter insufficiency and reflux esophagitis with resultant deep ulceration might also result in pyothorax.12 In case 1 of the present study, pyothorax was probably the result of gross nodule perforation coupled with aberrant migration of adult worms to the adventitia. In case 2, the esophageal rupture was very close to the nodule and the cardia, suggesting the possibility of severe reflux esophagitis-induced perforation of the esophagus.
Young adult and large-breed dogs, especially the German shepherd and Labrador breeds, are apparently more prone to spirocercosis, as was the case with the first dog in the present study (German shepherd) and the second dog (3-year-old).17 Both of these cases demonstrated historical or clinical evidence of esophageal dysphagia, manifested as odynophagia and/or regurgitation, in addition to clinical findings suggestive of pyothorax, such as weakness, depression and muffled heart sounds.4,5 Importantly, another study showed that five dogs with S. lupi-associated pyothorax 11 lacked evidence of esophageal dysphagia on admission, indicating that in geographic areas endemic of spirocercosis, the latter should be considered a differential of canine pyothorax, even in the absence of historical or clinical evidence referable to esophageal disease. Furthermore, case 2 of the current study presented with melena, which is uncommonly seen in canine spirocercosis, either as the sole presenting complaint or in the context of other signs of esophageal disease.4,5
A mild-to-moderate, non-regenerative anemia is expected in approximately 50% of dogs with spirocercosis due to the chronic inflammatory process, as in case 1 of this study.4,5 Uncommonly, a severe acute or chronic hemorrhagic anemia may occur if the benign or neoplastic masses become ulcerated, resulting in gross or occult blood loss (melena), as witnessed in case 2 of this study.14 Neutrophilic leucocytosis was detected in both dogs and a left shift was documented in case 1; these hematological alterations are more frequently seen in dogs with S. lupi-associated pyothorax and esophageal sarcomas rather than in those with benign parasitic nodules.4,5,11,14 Absolute monocytosis (case 1) is also a relatively common finding appearing in 25-40% of the affected dogs,4,5 along with increased serum alkaline phosphatase activity, hypoalbuminemia, hypoglycemia and hyperbilirubinemia as also noted in case 1 of this study. Elevated alkaline phosphatase activity has been consistently reported in case series of dogs with spirocercosis, although the specific enzyme isoform involved remains elusive. 4,5,14 Interestingly, alkaline phosphatase was recently found to be a poor marker of neoplastic transformation of esophageal nodules in canine spirocercosis.18 Since no gross liver disease was detected post mortem in case 1, the association of biochemical alterations with an underlying primary hepatopathy appears rather unlikely in this instance; it was most probably induced by septic inflammation as previously described.11
Diagnosis of spirocercosis can be challenging. In the present study, the ante mortem diagnosis was based on fecal examination, as the infection is highly endemic in this geographic area. Hence, a high index of suspicion is exercised in the clinical setting. Thoracic radiography was also helpful in case 1, in which a caudal thoracic mass was identified, raising suspicion of spirocercosis.5 Esophagoscopy is the diagnostic modality of choice for direct visualization of S. lupi nodules, but neither of the currently described dogs was subjected to this examination due to the owner’s decision for euthanasia in case 1 and the unexpected death of the animal in case 2. In consequence, the definitive diagnosis and establishment of the pathogenetic continuum between pyothorax and spirocercosis were exclusively based on post mortem examination.
The histopathological nature of the non-neoplastic esophageal lesions in canine spirocercosis is a matter of controversy. Historically, the esophageal nodules were thought, in the main, to represent granulomatous lesions.19 Recently, however, a large case series of non-neoplastic S. lupi-induced esophageal lesions showed that the inflammatory infiltrate was predominated by lymphocytes and plasma cells, rather than neutrophils and macrophages.20 In the present cases, the esophageal nodule histopathology pattern was consistent with pyogranulomatous or even granulomatous reaction, possibly suggesting that (pyo)granulomatous inflammation should be considered as a common response of canine esophagus to S. lupi-infection.
No treatment of S. lupi-associated pyothorax was undertaken in the present cases as the owner declined treatment in case 1 and case 2 died unexpectedly. Interestingly, in a series of five cases of S. lupi-associated pyothorax, four dogs were successfully treated with a combination of anthelminthic treatment (doramectin), broad-spectrum antibiotics and periodic thoracic lavage following thoracostomy tube placement, suggesting that conservative management of pyothorax may be considered in carefully selected cases.11
In conclusion, S. lupi infection should be included in the differential diagnosis of all canine pyothorax cases in highly endemic geographic areas, even in the absence of typical manifestations of esophageal spirocercosis at admission.
> References
1. Hawkins EC. Disorders of the pleural cavity. In: Small animal internal medicine. Nelson RW, Couto CG (eds). 4th edn. Mosby Elsevier: St. Louis, 2009, pp. 335-340.
2. Demetriou JL, Foale RD, Ladlow J, McGrotty Y, Faulkner J, Kirby BM. Canine and feline pyothorax: a retrospective study of 50 cases in the UK and Ireland. J Small Anim Pract 2002, 43: 388-394.
3. Lobetti RG. Survey of the incidence, diagnosis, clinical manifestations and treatment of Spirocerca lupi in South Africa. J S Afr Vet Assoc 2000, 71: 43-46.
4. Mazaki-Tovi M, Baneth G, Aroch I, Harrus S, Kass PH, Ben-Ari T, Zur G, Aizenberg I, Bark H, Lavy E. Canine spirocercosis: clinical, diagnostic, pathologic and epidemiologic characteristics. Vet Parasitol 2002, 107: 235-250.
5. Mylonakis ME, Rallis T, Koutinas AF, Leontides LS, Patsikas M, Florou M, Papadopoulos E, Fytianou A. Clinical signs and clinocipathological abnormalities in dogs with clinical spirocercosis: 39 cases (1996-2004). J Am Vet Med Assoc 2006, 228:1063-1067.
6. Van der Merwe LL, Kirberger RM, Clift S, Williams M, Keller N, Naidoo V. Spirocerca lupi infection in the dog: A review. Vet J 2008, 176: 294-309.
7. Lobetti R. Tropical diseases. In: Textbook of veterinary internal medicine. Ettinger SJ, Feldman EC (eds). 6th edn. Saunders Elsevier: St. Louis, 2005, pp. 699-701.
8. Mertens MM, Fossum TW, MacDonald KA. Pleural and extrapleural diseases. In: Textbook of veterinary internal medicine. Ettinger SJ, Feldman EC (eds). 6th edn. Saunders Elsevier: St. Louis, 2005, pp. 1272-1283.
9. Hawkins EC, Fossum TW. Pleural effusion. In: Kirk’s current veterinary therapy XIV. Bonagura JD, Twedt DC (eds). 14th edn. Saunders Elsevier: St. Louis, 2009, pp. 675- 684.
10. Chandrasekharon KP, Sastry GA, Menon MN. Canine spirocercosis with special reference to the incidence and lesions. Brit Vet J 1958, 114: 388-395.
11. Klainbart S, Mazaki-Tovi M, Auerbach N, Aizenberg I, Bruhim Y, Dank G, Lavy E, Aroch I, Harrus S. Spirocercosis-associated pyothorax in dogs. Vet J 2007, 173: 209-214.
12. Hamir AN. Oesophageal perforation and pyothorax associated with Spirocerca lupi infestation in a dog. Vet Rec 1986, 119: 276.
13. Jayawardena KLTD, Kularathna KDCP, de Silva DDN, Dangolla A. Spirocercosis-associated pyothorax in a dog: A case report. In: Congress proceedings of the Paradeniya University Research Sessions. Peradeniya, Sri Lanka, 2011, 16: pp. 113.
14. Ranen E, Lavy E, Aizenberg I, Perl S, Harrus S. Spirocercosisassociated esophageal sarcomas in dogs. A retrospective study of 17 cases (1997-2003). Vet Parasitol 2004, 19: 209-221.
15. Babero BB, Fawzi AH, Al-dabagh MA. Zoonoses in Iraq. Further studies on spirocercosis. Br Vet J 1965, 121: 183- 190.
16. Harrus S, Harmelin A, Markovics A, Bark H. Spirocerca lupi infection in the dog: aberrant migration. J Am Anim Hosp Assoc 1996, 32: 125-130.
17. Mylonakis ME, Rallis T, Koutinas AF. Canine spirocercosis. Compend Contin Educ Pract Vet 2008, 30: 111-116.
18. Mukorera V, van der Merwe LL, Lavy E, Aroch I, Dvir E. Serum alkaline phosphatase activity is not a marker for neoplastic transformation of esophageal nodules in canine spirocercosis. Vet Clin Pathol 2011, 40: 389-392.
19. Bailey WS. Spirocerca lupi: a continuing inquiry. J Parasitol 1972, 58: 3-22.
20. Dvir E, Clift SJ, Williams MC. Proposed histological progression of the Spirocerca lupi-induced oesophageal lesion in dogs. Vet Parasitol 2010, 168:71-77.



