> Abstract
Obesity ranks as the most common nutritional disorder reaching epidemic proportions among dogs and cats, at least in the developed world. Caused by energy intake exceeding energy loss, it results in adipose tissue accumulation in the organism negatively affecting an individual’s health. The list of hormones, neurotransmitters and substances secreted by adipose tissue itself, proven to have an active part in body weight modulation, is extensive. Predisposing factors contributing to the appearance of this disease include age, gender, breed and living conditions. The most common conditions to be associated with obesity are osteoarthritis, diseases of the cardiovascular and respiratory system, hypertension, hepatic lipidosis and type II diabetes mellitus. The evaluation and grading of obesity is based on the findings of observation and palpation of the animal, taking into consideration the predefined guidelines. The aim of any treatment plan applied in obesity management is the reduction of energy input and increase in output. This is mainly accomplished by reducing daily consumed calories and increasing physical activity. A fundamental requirement for successful management is that the owner understands the animal’s problem and is willing to cooperate with their veterinarian on a long-term basis. Finally, re-evaluation of body weight should occur at monthly intervals so that the dietary plan can be adjusted accordingly.
> Just what is obesity?
Obesity ranks as the most common nutritional disorder, reaching epidemic proportions among dogs and cats in most countries of the modern world. It is caused by dietary energy intake overcoming energy expenditure, visually depicted as calories stored in the form of fat.1 But what, in truth, is the definition of obesity? Obesity is defined as the accumulation of excessive body fat, resulting in a reversible impairment of natural body function, and hence a health disorder.2 Health impacts of obesity in companion animal medicine are similar to those of any other chronic disease, and for that reason it should receive the same attention and care.3
In humans, obesity is recognized as an excess of 20% to 25% above the ideal body weight. The same is approximately accepted in dogs and cats. A long-term study of dogs demonstrated that even a moderate increase in body weight can negatively influence the health status and life span of the animal.4
The prevalence of obesity in dogs and cats varies depending on the geographical origins of each survey. For example, in two validated surveys in the USA, 29-34% of dogs were classified as overweight and 5-8% as obese,5 whereas 19- 29% of cats were considered overweight and 6-8% obese.6 These percentages were higher for middle-aged animals, with both species aged from 5 to 10 years being almost 50% overweight or obese.5,6 Unfortunately, similar data concerning the prevalence of obesity in dogs and cats are unavailable in our country. However, according to the authors’ personal experience, the obesity issue does not appear to be of significance in small animals in Greece, notwithstanding the fact that according to statistic results, it ranks first as a nation in obesity prevalence rates in Europe. A preliminary review of the Internal Medicine department records of the Companion Animal Clinic, A.U.Th. revealed that the percentage of overweight/obese dogs admitted for various reasons from January to December 2011 was 11%, and the corresponding percentage in cats was 15%.
The aim of this study was to extensively review the role of obesity in various organ or system functions in dogs and cats and to present effective treatment plans.
> How does obesity develop?
It was previously theorized that the number of adipose tissue cells was defined in childhood and that the range of their triglyceride content was responsible for the development of obesity. Results of more recent studies, however, showed that the adipose tissue of an individual has a large population of stem cells and pre-adipocytes from where the body can “recruit” new adipocytes, should already existent ones show high levels of hypertrophy. 7 An important step in the clarification of the pathophysiology of obesity is understanding the mechanism behind an animal’s appetite.1 The hypothalamus, where hunger and satiety centers are located,8 has a key role as the main regulatory organ for modifying appetite.1 The aforementioned centers are regulated by various hormones, gastrointestinal peptides and other substances circulating in the bloodstream, as well as the central and autonomic nervous systems, that modify the animal’s appetite.1
Hormones with an active role in regulating appetite are cholecystokinin, leptin, ghrelin, pancreatic peptide YY, adiponectin etc.1 Each of them acts through a different mechanism such as stimulating hunger, increasing satiety or activating energy expenditure. More specifically, cholecystokinin, secreted in the duodenum, is mainly intended to activate bile secretion and suppress appetite, whereas leptin, secreted by adipocytes, is considered to increase satiety3, thereby contributing to the reduction of food intake and promotion of energy loss.1 Ghrelin is produced by the stomach epithelial lining and is responsible for increasing appetite. For that reason, it has been called “the hunger hormone”. Finally, pancreatic peptide YY appears to maintain high levels of satiety.1
Appetite is also regulated by various neurotransmitters like serotonin, norepinephrine and dopamine, 8 which can be stimulated by multiple factors like consistency, palatability and sight of food, as well as stomach filling and stress.
Finally, sympathetic and parasympathetic nervous system activity regulates uptake, storage and mobilization of energy from the fatty tissue, liver or muscle.8
The most common way to create obesity is for the dietary intake to exceed body requirements in amount or caloric density; combined with the lack of any significant physical activity, this results in the storage of excessive energy in the form of triglyceride content in adipose tissue cells.1 The interaction between genetic and environmental factors is considered to be the general root cause of obesity.9 Rarely, miscellaneous metabolic disorders can lead to obesity. One such example is canine hypothyroidism, in which low serum thyroxine concentrations decrease metabolic rate.3 In hyperadrenocorticism, fat redistribution and storage is observed mainly in the trunk and especially in the abdominal cavity and tail base. Due to this redistribution, a false impression is generated that the animal’s body weight has increased whereas, in truth, only less than 50% of hyperadrenocorticism cases present with actual weight gain. Weight gain reflects the increase in adipose tissue caused by hyperphagia, resulting from suppression of the ACTH secretory factor in the hypothalamus by a higher concentration in glucocorticosteroids. Hyperinsulinemia in cases of insulinoma or insulin overdose in diabetic patients may cause hyperphagia, apparently due to concurrent hypoglycaemia. Increased blood levels of growth hormone in cases of acromegaly lead to an increase in connective and bone tissue mass resulting in an obese appearance. Nevertheless, given that the growth hormone can also cause hyperphagia, an increase in adipose tissue is noted in these animals, thus leading to obesity.6
Predisposing factors associated with obesity include age, gender, breed and living conditions. Obesity is more common in middle-aged and geriatric animals.3 Under normal conditions, a reduction in muscle mass and exercise is progressively observed in these age groups, which contributes to adiposity.8
Obesity is usually observed in spayed female dogs and neutered male cats. A genetic predisposition to obesity has been studied in dogs. It is reported that obesity is highly associated with some dog breeds, such as Golden retrievers, Dachshunds, Shetland sheepdogs, Labrador retrievers, Cocker spaniels, Cavalier King Charles Spaniels, Beagles and Dalmatians.3 No clear predilection has been reported regarding cat breeds. On the contrary, Siamese and Abyssinian cats tend to appear thinner than cats belonging to other breeds.8
Other predisposing factors for obesity include mild and “sedentary” lifestyles with decreased activity levels, as well as ad libitum feeding regimens of high caloric density.3
One of the most important predisposing factors, considered by some researchers as causative, is neutering.3 In one study, increased food intake and reduced metabolic rate were noted after neutering/spaying3 and resulted in weight gain,10 whereas in another study the effect of neutering/ spaying in food consumption was not substantiated. 11 However, estrogen and progesterone blood concentrations in a dog during anestrus are similar to those of a spayed female dog; therefore, there is no particular medical reason to avoid ovariohysterectomy.
>What are the results of obesity?
Until recently, adipose tissue was considered to be a simple, physiologically inert energy source.3 However, it was swiftly proven that, in truth, it is a complicated active endocrine organ, producing cytokines called adipokines.7 These peptides are secreted exclusively by adipocytes. No other cell lines of adipose tissue such as macrophages or neutrophils contribute to their production.2 Up until now, more than 50-100 kinds of adipokines have been identified in animals and humans, such as leptin, resistin, adiponectin, various interleukins including IL-1b, IL-6, IL-10 and IL-18, other cytokines like tumor necrosis factor alpha (TNF-a) and transforming growth factor-b (TGFb), chemokines, and acute phase proteins such as serum amyloid A (SAA), C-reactive protein (CRP), metallothionein etc.1,3 Adipokines are essential to diverse biological processes causing inflammation, as well as glucose imbalance, insulin resistance, immune function, fluid balance, blood vessel integrity, blood cell formation and cell multiplication. 7,12
Hormones secreted by adipocytes that have been studied the most are leptin, adiponectin,13 and the renin-angiotensin system.7 Secretion of leptin is relative to adipose tissue mass, and is thought to reduce appetite, increase energy expenditure, and possess immunogenic and neuroendocrine properties.14 However, a number of obese people develop leptin resistance. Adiponectin enhances sensitivity of body tissues to insulin, and its concentration levels rise with insulin activity and drop when adipose tissue increases.3,14 Beyond its anti-diabetic and anti-inflammatory properties, it inhibits the development of atherosclerosis15 and also appears to improve cardiac function in patients with myocardial infarction and suppress the development of cancer.7 Thin dogs show a higher concentration of adiponectin compared with people in whom, in contrast, there is no decrease with fat deposition. Considering that dogs do not usually develop type II diabetes mellitus, it may be concluded that adiponectin may have a protective role in this animal species.16 Finally, obesity in dogs promotes increased activity of the reninangiotensin system, which contributes to the development of localized inflammation in adipose tissue, deterioration of metabolic syndrome, and insulin resistance.7
Obesity is associated with a state of chronic lowgrade inflammation localized in adipose tissue and also with disseminated inflammation.7,17 In obese people, concentrations of various inflammatory markers (CRP, IL-6, TNF-α) increase, only to decrease following weight loss.18 Some inflammatory markers in people increase the risk of metabolic syndrome and diabetes mellitus type II, mainly by suppressing insulin receptor activity, 19 whereas IL-10 possesses anti-inflammatory properties by reducing concentration levels of proinflammatory mediators like TNF-α.2 In adipose tissue, monocyte chemoattractant protein-1 is produced (MCP-1) which attracts monocytes, further contributing to the development of inflammation and release of other cytokines.20 The liver is also implicated in the whole process of inflammation by activating Kupffer cells and producing inflammatory mediators, thereby attracting other inflammatory cells.21
Obesity is associated with important changes in the quality and quantity of insulin secretion and in the suppression of insulin activity, thus causing resistance.22 The exact mechanism of insulin resistance has not yet been elucidated.1 However, in most cases, these changes are reversible with weight loss.2 One study of cats showed that further acquisition of 1kg body weight causes 30% reduction of sensitivity to insulin.23 The possibility of diabetes mellitus developing in an obese cat compared to a cat of normal body weight is 6-8 times greater, whereas the risk is redoubled in dogs. Suppression of sensitivity to insulin increases lipolysis resulting in an increase in concentration of free fatty acids which through free radicals Ο+, ΝΟ+ and ΟΗ- further add to insulin resistance.1 Free fatty acids contribute to the development of oxidative stress which, in cases of obesity, constitutes another cause of insulin resistance.24 It was also discovered that obesity modifies insulin secretion. 1 Insulin resistance reflects the inability of tissues to respond normally, leading to counteracting insulin secretion increase by the pancreas. In obese animals, although blood glucose concentration is still maintained at normal levels, insulin secretion rates have already changed, mainly due to persistent high levels of the latter during the second phase of its release in the bloodstream, resulting in hyperglycemia in some cases.1 The end result of prolonged hyperglycemia in cats is impaired secretory ability of Langerhans islet pancreatic b-cells due to glucose toxicity and blood triglycerides, and eventually type II diabetes mellitus, which is not frequently observed in dogs.25
Under normal circumstances, insulin crosses the blood-brain barrier and acts on the hypothalamus to decrease appetite.26 In cases of insulin resistance, this mechanism is suppressed. In contrast, insulin resistance in combination with hyperinsulinemia increases hunger, reducing lipolysis and also reducing energy expenditure in the form of heat production after the meal, resulting in further difficulty in reversing obesity.27
Given the anti-inflammatory, antithrombotic and vasodilating properties of insulin, insulin resistance observed in obesity causes miscellaneous health disorders.7 Insulin resistance has been found to contribute to the development of multiple human diseases, such as cardiovascular disorders, mammary tumors, prostate or large intestine tumors, as well as disorders of the kidneys or liver. 28 With the exception of diabetes mellitus, most of these disorders have not been substantiated in dogs and cats. Insulin resistance also plays a role in “preserving” obesity through energy imbalance.
In some obese dogs and cats, insulin resistance has been observed in combination with hyperinsulinemia. Most diabetic dogs present with type I diabetes mellitus. However, in type I diabetic dogs with the presence of comorbid disorders, such as obesity or hyperadrenocorticism, insulin resistance can occur. In contrast, a large number of cats, but also people, may be affected by type II diabetes mellitus due to obesity. In these cats, weight loss contributes to the modulation of glucose levels in the bloodstream and in the reduction or even obliteration of requirements in externally administered insulin and/or oral hypoglycaemic drugs.8 Studies in dogs and cats revealed that subcutaneous and visceral adipose tissue do not appear to have an important role in the development of insulin resistance.29,30 On the contrary, visceral fat in humans has a positive correlation with the appearance of insulin resistance.22
Furthermore, in obese euthyroid animals, an increase in free T4 and T3 blood levels has been observed in cats and dogs respectively.31Obese hypothyroid dogs appear to have decreased total and free T4 and increased TSH concentrations, which illustrates that obesity does not affect the diagnosis of hypothyroidism in dogs. In addition, cats displayed higher levels of Langerhans islet amyloid polypeptide (amylin), a polypeptide hormone secreted simultaneously with insulin from b-cells of pancreatic islets of Langerhans, and a precursor of islet amyloid. Nonetheless, obesity does not seem to affect the risk of amyloidosis in cats.1 Obese animals and humans have increased concentrations of triglyceride in the form of very low density lipoproteins (VLDL). Increased concentration of VLDL may be related to atherosclerosis, coronary disease and hypertension in humans, 32 but it does not appear to cause similar health disorders in cats, at least not in those presenting with obesity for a short length of time.22 On the other hand, increased cholesterol concentration in dogs is related to atherosclerosis and hypertension. 33
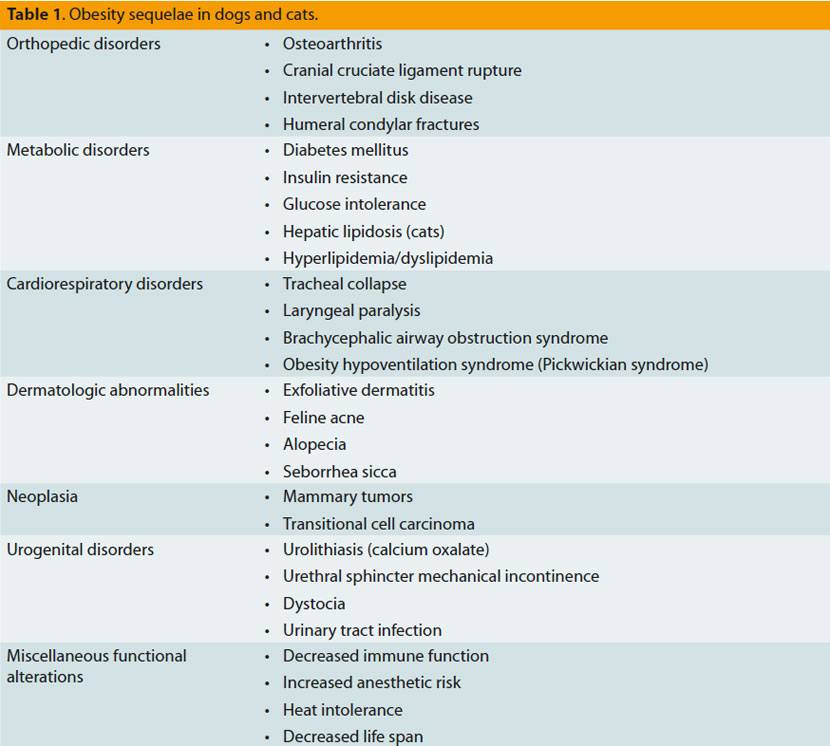
The quality of life of an obese dog appears to be affected to varying degrees, and this is expressed as a vitality and mentality disorder, or even as chronic body stress combined with pain.34 Generally, however, the consequences of obesity in dogs and cats are multiple, as presented in table 1. The most important among them are the following:
Osteoarthritis: Obesity contributes to the deterioration of arthropathies by increasing the forces exerted upon the joints resulting in articular cartilage degeneration.8
Cardiovascular disorders: The increase in adipose tissue leads to an increase in perfused tissue mass, resulting in a steep increase in the cardiac workload through increases in blood volume and pulse volume. Obese individuals develop hypertrophy of the myocardium and heart dilation, with a simultaneous high risk of congestive heart failure.8
Respiratory disorders: Extensive intrathoracic and visceral fat cause a corresponding disorder in pulmonary expansion and diaphragm hypocontractility, respectively. In addition, excessive adipose tissue requires further cardiorespiratory effort. It is important to mention that obesity may, through aforementioned mechanisms, cause deterioration of a preexisting respiratory disease.35 Obesity in humans is related to sleep apnea and asthma.2
Hypertension: Obesity-related hypertension is observed only in humans and experimental studies in dogs,8 and it is caused by an increase in angiotensinogen blood concentration.2
Hepatic lipidosis: Although obesity is a predisposing factor for hepatic lipidosis in cats, the pathophysiologic connection has not been fully elucidated since this abnormal condition also develops in cats with a normal or even lower body weight.8
>Obesity: friend or foe?
Notwithstanding all the aforementioned, several studies, particularly those in humans, have shown that obesity seems to play a beneficiary role in the evolution of various disorders. This fact is cited as the “obesity paradox” and it has been observed in chronic renal failure, heart failure, rheumatoid arthritis and chronic obstructive lung disease.36-40
An explanation for this interesting phenomenon has not yet been given. However, several theories have been proposed. This is most likely a multifactorial process in which adipokines, various neurohormonal changes and pharmaceutical substances administered in the management of any comorbid disease may play a role. It is considered an important fact that the amount of muscle mass is greater in obese individuals than in any other body type, especially the emaciated type. Hence, the added muscle mass of obese people provides greater energy storing potential for the organism toward the management of a catabolic disease.41
The survival time of dogs and cats with heart failure was longer in animals with increased body weight compared to those who were lean or emaciated. 42,43 A similar observation was made in dogs with chronic renal failure.44 In consequence, the results of these studies, and many others, form the foundations which define the importance of body weight, especially in the form of muscle mass, in the course of a catabolic disease.
> Evaluating an obese animal
Obesity is generally easily recognized in dogs and not mistaken for any other disease causing abdominal distension. On the contrary, in cats obesity is more difficult to recognize, and an accurate diagnosis is based on the accumulation of large amounts of fat in the abdominal and inguinal area. In both animal species, however, differentiation between primary and secondary obesity is difficult. To evaluate body condition in dogs and cats, several scoring systems have been formulated with variable objectivity, practicality and effectiveness. 3 The Body Condition Scoring (BCS) scale is a simple, widely used, but subjective method to evaluate an animal’s physical condition; nonetheless, it does have reproducibility.
In order to apply this scoring system, observation and palpation of the animal are necessary.45 It is of greater value compared to body weight because the latter does not evaluate differences in percentages of muscular and adipose tissue in the body. Applying BCS may be difficult in animals that have recently lost weight or have long hair coats.1 Three scoring systems have been formulated, according to the number of points they contain (5, 7, or 9), and their selection is based on subjective criteria.45 The 9-point scale appears to be preferable in the daily clinical setting.1 The aim of this scoring system was to create a communication code through the arithmetic expression of the percentage of animal’s body fat (Image 1).45 In the 9-point scale, 5 is assigned to the ideal physical condition in a cat, and 4 or 5 to the ideal dog. In all three systems, scores of 1 to 3 suggest an underfed pet. Finally, a score in cats ranging from 7 to 9 suggests an overfed animal, whereas corresponding canine points range from 6 to 9.1
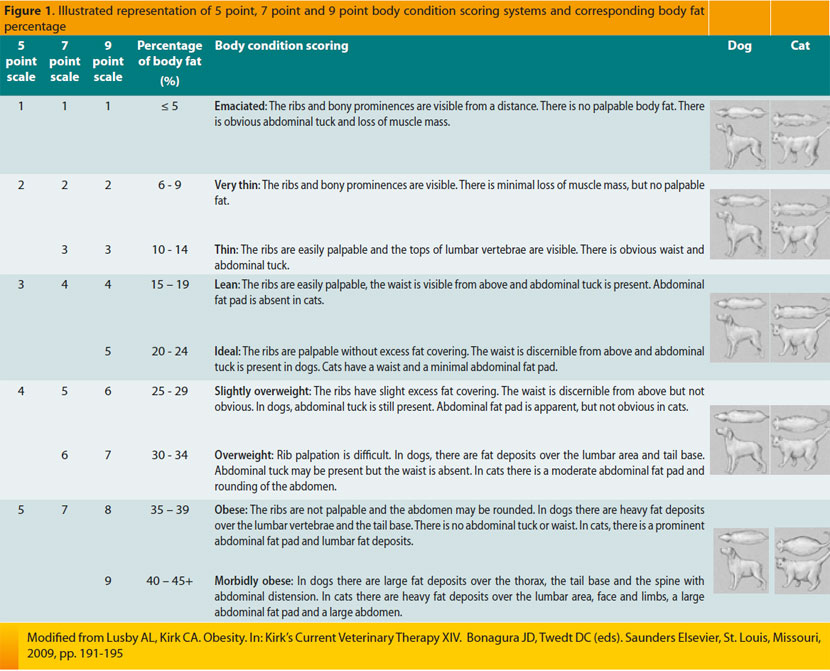
The body mass index (BMI), which has been used after the validation of a special formula for this exact purpose, has been extensively employed in humans, and it is an objective measure in order to evaluate adipose tissue percentage in the body. The following formula was established to estimate BMI in cats:
BMI = body weight (kg) / [body length (m) × height (m)]
Height corresponds to the distance measured from the withers through the elbow to the proximal end of the metacarpal bone, whereas body length is measured as the distance from the shoulder to the ischial tuberosity.46 Another method applied in evaluating the amount of adipose tissue in the body is body perimeter measured exactly behind the last rib. To calculate BMI and body perimeter, special equipment is not necessary.22 On the contrary, the latter is required for other methods like magnetic resonance imaging (MRI) or dualenergy x-ray absorptiometry (DEXA), which calculate the exact amount of adipose tissue stored in the body.1
> Solving the obesity problem
Presumed that it is a case of primary and not secondary obesity, the answer to the question «..what is to be done now ?...” seems to be relatively simple. 1 The concept behind weight loss measures in dogs and cats involves the reduction of caloric intake and increase of energy expenditure.22 This is accomplished by reducing daily consumed calories and increasing physical activity.3 Even though the review of obesity treatment within these few lines could almost have been complete, managing each individual case is so complicated as to necessitate tailored treatment; general measures are but an umbrella under which lie all the small but important details, each of which ultimately constitutes a particular therapeutic measure. Gathered together, all those small details make up the animal’s living conditions, not only in terms essentially of the pet itself, but also of the people that comprise its immediate and wider surroundings. Initially, however, it is necessary for the veterinarian to evaluate whether the owner understands the animal’s problem and whether they possess the qualities essential to cooperation.22 Many times it is difficult to convince the owner that their animal is essentially ill, when they believe that it is in every way healthy;47 this becomes even more difficult when they are overweight or obese themselves.9 A recent study reported a correlation between the physical condition of the owner and that of the dog, whereas the same did not apply to cats.9 These are just some clues that could direct a veterinarian to different pathways of managing obesity.
Initially, it is mandatory for the veterinarian to explain in detail and confirm that the owner understands the disorder their animal is facing and any potential health sequelae that may occur.22 The interview that follows will have to include general questions at first, which will probe deeper, depending on the replies of the owner and the experience of the veterinarian. In the main, questions will have to pertain to the lifestyle and way of feeding the animal, living conditions, family members, their habits, and their relationship with the pet; whether it is fed by the owner, another family member or possibly everyone or whether there is a chance that the neighbors are feeding it; whether it goes out for unsupervised walks, hence enabling it to consume food of which they are unaware; if there are other animals living with the one in question; and what kind of food, composition and type does the animal prefer, the way and time of supplying meals, the amount of daily ingested food, and the number of daily meals, as well as the habit of treats.3 Further questions need to be asked related to the daily physical activity of the dog or cat, and the time taken devoted by the owner to walking and/or playing with their pet. The positive outcome of this interview mainly depends on the personality of the owner.22 Veterinarians should be prepared for the event of an owner displaying irritation at such questions if they are unable to understand their purpose.22,25
The daily amount of consumed food necessary to achieve weight loss is defined by BCS or current body fat percentage, ideal body weight for the animal (target weight) and the length of time during which the pet must reach the desired body weight. The time needed to accomplish the ideal body weight is calculated easily through the unbroken rule that the rate of weekly weight loss must not surpass 1-1.5% of the animal’s total body weight22 in order to minimize the feeling of hunger, avoid loss of muscular tissue, and diminish the risk of swift regain of weight after the end of treatment.45 Indeed, the faster the rate of weight loss, the swifter the reuptake of weight following completion of the treatment plan.47 A realistic plan would be to gradually reach ideal body weight through incremental weight loss which each time amounts to 15% of the animal’s body weight at the time. Particular attention should be given to the rate of weight loss in cats, due to its cause-andeffect relationship with liver lipidosis.3,22 Calculating ideal body weight can be done through data related to each breed and veterinarian experience. A simple way for a veterinarian to calculate ideal body weight for a dog or cat is to keep records of body weight and note any deviations during the animal’s life. Ideal weight is defined as the weight an animal acquires at the time of reaching adulthood, estimated for most animals at about the first year of age.3
Having determined the ideal body weight, a dietary plan and an exercise program must be implemented.
Dietary composition and type is one more topic of discussion with the owner.3 Τhere are three basic categories: special market weight loss diets, reducing the daily amount of the animal’s current diet and especially prepared people-intended food of a particular composition. The first option is preferable because weight loss by special market foods is guaranteed,47 but this does not exclude good results if the other two diets are followed. Regarding people-intended food, there are special recipes that can be used by the owner, however simultaneous administration of dietary supplements is required. Something similar applies if the quantity of a maintenance diet is simply reduced, implying the risk of developing deficiencies of necessary nutrients due to a reduced daily intake.3
In general, composition of weight-reduction diets may be based on two different theories. It is commonly estimated that diets of reduced caloric density should be recommended.1 The first theory is based on the belief that high-protein, low-fat, low-carbohydrate diets lead to loss of fat and preservation or possibly an increase in muscle mass.47 This particular theory essentially supports that the supply of energy in the form of protein causes greater food-generated heat production compared with carbohydrate and fat.48 On the contrary, the second theory advocates weight loss through low-protein, low-fat, low-carbohydrate and high-fiber diets which are conducive to increased satiety and decreased food consumption. 49 A fact of particular importance is that in obese individuals, intestinally absorbable dietary fiber reduces insulin resistance. Initially, aversion to this particular diet may occur, and it is managed by gradual transition from previously fed diets to the prescribed weight loss diet. A recent study found that dogs fed a high-protein, high-fiber diet consumed even smaller amounts of food.50 Naturally, evaluating the results of this study was no easy task due to different fiber origin in compared diets. Undeniably, these two theories raised more questions in order to ensure the safe administration of representative diets to cats. What does occur is that selecting a high-protein, low-carbohydrate diet or a high-fiber diet is purely a matter of personal preference because the essential role in weight loss belongs to caloric restriction.3 Finally, according to results of clinical studies in cats and dogs, the beneficial influence of omega-3 fatty acids, carnitine and isoflavonoids found in weight loss diets seems to be of particular importance.45
For daily energy requirements to be estimated, a simple formula is applied. In order for that to be understood, a few particular concepts must be explained. Energy required to cover daily needs is called daily energy requirement (DER) and it is comprised of various energy components, mainly resting energy requirement (RER) and exercise energy requirement (EER).3 RER expresses the basic metabolic rate and typically accounts for 60- 80% of total DER, whereas EER is energy exerted through exercise and accounts for 10 to 20% of DER. In order to calculate RER, metabolically inactive fat mass must be subtracted from the animal’s total body weight. Therefore, using ideal body weight is mandatory. The formula used to estimate RER is:
RER= 70 (BWkg 0,75)
For an easier calculation a linear equation follows, however the latter applies to animals over 2 kg:
RER= (30×BWkg)+70
Finally, DER is calculated by the following formula:
DER= 1 – 1,2 × RER (dog)
DER= 0,8 × RER (cat)
The result is the total daily amount of calories that should be fed to a dog or cat over a 24-hour period in order for it to lose weight.3 With a view to assisting veterinarians, special software has been created to help calculate the daily intake of food. It is important to note that in female dogs, the amount of ingested food should be higher than that intended for males.47 The way of offering food depends on the individual animal’s body type. Thus, food can be offered in 2-3 daily meals or the entire daily amount administered to be gradually eaten by the pet in small increments. In the event of a household having more than one pet, then the overweight animal should be fed separately from the rest. Alternative solutions that are more likely to apply to cats include placing the food of other cats on higher surfaces, rendering it impossible for the obese cat to reach because it cannot easily leap as high, or to place the food in a large crate with a hole through which the obese cat cannot pass. Parallel to the reduction of daily consumed calories, exercise-induced energy expenditure is considered to be of particular importance.47 One study noted that dogs with increased physical activity consuming 20% more calories presented a similar rate of weight loss to that of those with reduced physical activity.51 Exercise appears capable of reversing the metabolic imbalance causing obesity, even in the absence of significant weight loss.47 Exercise includes establishing walking, playing, training or other forms of physical activity. Sufficient canine exercise rates for a dog include 20-30-minute walks, three to four times a week or a 10-minute increase in total daily exercise. As concerns cats, physical activity can be expressed through playing. Veterinarians should discuss the matter extensively with the owners and provide them with all the relevant information concerning the available options, in an endeavor to arouse their resourcefulness. Reported examples are environmental enrichment for cats with more toys, placement of food on surfaces that require effort to reach, hiding kibble or treats in various places or inside toys resulting in increased physical activity in order to extract, it etc. The above should be adjusted accordingly to pet and owner lifestyle; any coexisting health issues concerning either of them should be seriously taken into account (e.g. osteoarthritis).3 By and large, however, increased physical activity and environmental enrichment are beneficiary for both pets and owners.47
Various pharmaceutical substances have been tested for weight loss in people. Their effectiveness is variable, and the aim is to increase satiety, bind and block intestinal absorption of nutrients, increase metabolic rate and, finally, redistribute energy stored in adipose tissue to more metabolically active tissue such as muscle. Two drugs approved for weight loss in people are sibutramine and orlistat; the former is a serotonin and norepinephrine reuptake inhibitor that produces increased satiety and stimulation of heat production, while the latter contains a pancreatic lipase inhibitor that increases fecal fat excretion by preventing its digestion and absorption.47 Administration of these drugs is discouraged in cats. The results of an orlistat trial in dogs proved modest. Reported side effects included mild fecal incontinence. 3
Recently, two pharmaceutical substances were introduced for canine weight loss induction: dirlotapide and mitratapide. These substances prevent intraluminal fat absorption from intestinal tissue. Dirlotapide may be administered at a dose of 0.01-0.2 ml/kg up to three times daily depending on animal response. Mitratapide is found on the market in containers on which the required dose is written according to body weight at the time. Treatment is administered for three weeks and interrupted for two weeks, after which the dog’s dietary needs are re-evaluated, the daily amount of food is adjusted and mitratapide is administered for three more weeks.1
The key to any weight loss treatment for dogs or cats lies in the persistence and patience of both the veterinarian and owner, preserved through close communication and simultaneous monitoring of the result.3 Monthly body weight reevaluations of the pet and adjustments in the dietary plan aiming for further weight loss are considered to be of particular importance.47 It should be noted that the weight loss program is applied in a time frame of months or even years, which is defined by the rate of weight loss, animal response and owner compliance. Initial failures are not rare; rather than achieving a decrease or preservation of the animal’s weight, the latter may be found to increase. In such cases, it is mandatory to methodically re-evaluate the dietary and exercise schedule, as well as the owner’s honesty regarding whether or not veterinary advice was followed faithfully. In the event of certain indiscretions being found, these are corrected; otherwise, the dog’s and cat’s dietary schedule is followed even more strictly. Until the desirable rhythm of weight loss is accomplished, a reduction of daily consumed calories by 10% every two weeks may be necessary.3 Finally, it must be clarified that the intended treatment plan must not, under any circumstances, be undesirable to the owner or animal, otherwise its implementation may disrupt their relationship.47
In conclusion, it must be noted that obesity is a disease with significant deleterious effects on animal health. Owners should thoroughly consider the animal’s problem and closely cooperate with veterinarians, with the aim of achieving a swift and radical correction of this condition.
> Bibliography
1. Hoenig M. Obesity. In: Clinical endocrinology of dogs and cats. Rijnberk A, Kooistra HS (eds). 2nd edn. Schlϋtersche Verlagsgessellschaft mbH & Co. KG, Hannover, 2010, pp. 297-302.
2. German AJ, Ryan VH, German AC, Wood IS, Tayhurn P. Obesity, its associated disorders and the role of inflammatory adipokines in companion animals. Vet J 2010, 185: 4-9.
3. Lusby AL, Kirk CA. Obesity. In: Kirk’s Current Veterinary Therapy XIV. Bonagura JD, Twedt DC (eds). Saunders Elsevier, St. Louis, Missouri, 2009, pp. 191-195.
4. Kealy RD, Lawler DF, Ballam JM, Mantz SL, Biery DN, Greeley EH, Lust G, Serge M, Smith GK, Stowe HD. Effects of diet restriction on life span and age-related changes in dogs. J Am Vet Med Assoc 2002, 220: 1315-1320.
5. Lund EM, Armstrong PJ, Kirk CA, Klausner JS. Prevalence and risk factors for obesity in adult dogs from private US veterinary practices. Inter J Appl Res Vet Med 2006, 4(2): 177-186.
6. Lund EM, Armstrong PJ, Kirk CA, Klausner JS. Prevalence and risk factors for obesity in adult cats from private US veterinary practices. Inter J Appl Res Vet Med 2005, 3(1): 88-96.
7. Radin MJ, Sharkey LC, Holycross BJ. Adipokines: a review of biological and analytical principles, and an update in dogs, cats and horses. Vet Clin Path 2009, 38: 136-156.
8. German AJ. Obesity biology and management. In: Textbook of Veterinary Internal Medicine. Ettinger SJ, Feldman EC (eds). 7th ed. Saunders Elsevier, St. Louis, Missouri, 2010, pp. 121-124.
9. Nijland ML, Stam F, Seidell JC. Overweight in dogs, but not in cats, is related to overweight to their owners. Public Health Nutr 2009, 13: 102-106.
10. Concannon PW, Meyers-Wallen VN. Current and proposed methods for contraception and termination of pregnancy in dogs and cats. J Am Vet Med Assoc 1991, 198(7): 1214-1225.
11. Salmeri KR, Bloomberg MS, Scruggs SL, Shille V. Gonadectomy in immature dogs: effects on skeletal, physical, and dehavioral development. J Am Vet Med Assoc 1991, 198(7): 1193-1203.
12. German AC, German AJ, Wood IS, Hunter L, Morris PJ, Trayhurn P. Development and optimization of a primary cell culture system for feline adipocytes. In: Proceedings of the 2009 British Small Animal Veterinary Association Congress. Birmingham, UK pp. 380- 381.
13. Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans 2005, 33: 1078-1081.
14. Wynne K, Stanley S, McGowan B, Bloom S. Appetite control. J Endocrinol 2005, 184: 291-318.
15. Kershaw E, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004, 89: 2548-2556.
16. Verkest KR, Rand JS, Fleeman LM, Morton JM, Richards AA, Rose FS, Whitehead JP. Distinct adiponectin profiles might contribute to differences in susceptibility to type 2 diabetes in dogs and humans. Domest Anim Endocrinol 2011, 41: 67-73.
17. Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of the white adipose tissue. Brit J Nutr 2004, 92: 347-355.
18. Manco M, Fernandez-Real JM, Equitani F, Vendrell J, Valera Mora M, Nanni G, Tondolo V, Calvani M, Ricart W, Castagneto M, Mingrone G. Effect of massive weight loss on inflammatory adipocytokines and the innate immune system in morbidly obese women. J Clin Endocrinol Metab 2007, 92: 483-490.
19. Spranger J, Kroke A, Moblig M, Hoffman K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003, 52: 812-817.
20. Singla P, Bardoloi A, Parkash AA. Metabolic effects of obesity: a review. World J Diabetes 2010, 1: 76-88.
21. Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006, 116: 1793-1801.
22. Hoenig M. Metabolism, diet and obesity. In: Consultation in feline internal medicine. August JR (ed). Vol 6. Saunders Elsevier, St. Louis, 2010 pp. 98-103.
23. Hoenig M, Thomaseth K, Brandao J, Waldron M, Ferguson DC. Assessment and mathematical modeling of glucose turnover and insulin sensitivity in lean and obese cats. Domest Anim Endocrinol 2006, 31: 373-389.
24. Subauste AR, Burant CF. Role of Fox01 in FFA-indused oxidative stress in adipocytes. Am J Physiol Endocrinol Metab 2007, 293: E159-E164.
25. Hoenig M, Hall G, Ferguson D, Jordan K, Henson M, Johnson K, O’Brien T. A feline model of experimentally induced islet amyloidosis. Am J Pathol 2000, 157: 2143-2150.
26. Wynne K, Stanley S, McGowan B, Bloom S. Appetite control. J Endocrinol 2005, 184: 291-318.
27. Watanabe T, Nomura M, Nakayasu K, Kawano T, Ito S, Nakaya Y. relationships between thermic effect of food, insulin resistance and autonomic nervous activity. J Med Invest 2006, 53: 153-158.
28. Ota T, takamura T, Kurita S, Matsuzawa N, Kita Y, Uno M, Akahori H, Miso H, Sakurai M, Zen Y, Nakanuma Y, Kaneko S. Insulin resistance accelerates a rat model of nonalcocholic steatohepatitis. Gastroenterol 2007, 132: 282-293.
29. Kim SP, Ellmerer M, Van Kitters GW, Bergman RN. Primacy of hepatic insulin resistance in the development of the metabolic syndrome indused by an isocaloric moderate-fat diet in the dog. Diabetes 2003, 52: 2453-2460.
30. Hoenig M, Thomaseth K, Waldron <. Ferguson DC. Insulin sensitivity, fat distribution, and adipocytokine response to different diets in lean and obese cats before and after weight loss. Am J Physiol Regul Integr Comp Physiol 2007, 292: R227-R234.
31. Ferguson DC, Caffall Z, Hoenig M. Obesity increases free thyroxine proportionally to nonesterified fatty acid concentrations in adult neutered female cats. J Endocrinol 2007, 194: 267-273.
32. Avramoglu RK, Basciano H, Adeli K. Lipid and lipoprotein dysregulation in insulin resistant states. Clin Chim Acta 2006, 368: 1-19.
33. Rocchini AP, Mao HZ, Badu K, Marker P, Rocchini AJ. Clonidine prevents insulin resistance and hypertension in obese dogs. Hypertension 1999, 33: 548-553.
34. German AJ, Holden SL, Wiseman-Orr ML, Reid J, Nolan AM, Biourge V, Morris PJ, Scott EM. Quality of life is reduced in obese dogs but improves after successful weight loss. Vet J 2012, 192(3): 428-434.
35. Manens J, Bolognin M, Bernaerts F, Diez M, Kirschvink N, Clercx C. Effects of obesity on lung function and airway reactivity in healthy dogs. Vet J 2012, 193(1): 217-221.
36. Port FK, Ashby VB, Dhingra RK, Roys EC, Wolfe RA. Dialysis dose and body mass index are strongly associated with survival in hemodialysis patients. J Am Soc Nephrol 2002, 13: 1061-1066.
37. Escalante A, Has RW, del Ricon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis. Arch Intern Med 2005, 165: 1624-1629.
38. Marti S, Munoz X, Rios J, Morell F, Ferrer J. Body weight and comorbidity predict mortality in COPD patients treated with oxygen therapy. Eur Pespir J 2006, 27: 689-696.
39. Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis 2007, 49: 581-591.
40. Kapoor JR, Heidenreich PA. Obesity and survival in patients with heart failure and preserved systolic function: A U-shaped relationship. Am Heart J 2010, 159: 75-80.
41. Wells JCK, Fewtrell MS, Williams JE, Haroun D, Lawson MS, Cole TJ. Body composition in normal weight, overweight and obese children: Matched case-control analyses of total and regional tissue masses, and body composition trends in relation to relative weight. Int J Obes 2006, 30: 1506-1513.
42. Slupe JL, Freeman LM, Rush JE. Association of body weight and body condition with survival in dogs with heart failure. J Vet Intern Med 2008, 22: 561-565.
43. Finn E, Freeman LM, Rush JE, Lee Y. The relationship between body weight, body condition and survival in cats with heart failure. J Vet Intern Med 2010, 24: 1369-1374.
44. Parker VJ, Freeman LM. Association between body condition and survival in dogs with acquired chronic kidney disease. J Vet Intern Med 2011, 25: 1306-1311.
45. Roudebush P, Schoenherr WD, Delaney SJ. An evidencebased review of the use of nutraceuticals and dietary supplementation for the management of obese and overweight pets. J Am Vet Med Assoc 2008, 232: 1646-1655.
46. Nelson RW, Himsel CA, Feldman EC, Bottoms GD. Glucose tolerance and insulin response in normal weight and obese cats. Am J Vet Res 1990, 51: 1357-1362.
47. Roudebush P, Schoenherr WD, Delaney SJ. An evidencebased review of the use of therapeutic foods, owner education, exercise, and drugs for the management of obese and overweight pets. J Am Vet Med Assoc 2008, 233: 717-725.
48. Leidy HJ, Mattes RD, Campbell WW. Effects of acute and chronic protein intake on metabolism, appetite, and ghrelin during weight loss. Obesity 2007, 15: 1215-1225.
49. Bosch G, Verbrugghe A, Hesta M, Holst JJ, vander Poel AF, Janssens GP, Hendriks WH. The effects of dietary fiber type on satiety-related hormones and voluntary food intake in dogs. Brit J Nutr 2009, 102: 318-325.
50. Weber M, Bissot T, Servet E, Sergheraert R, Biourge V, German A. A high-protein, high-fiber diet designed for weight loss improves satiety in dogs. J Vet Intern Med 2007, 21: 1203- 1208.
51. Wakshlag JJ, Struble AM, Warren BS, Maley M, Panasevich MR, Cummings KJ, Long GM, Laflamme DE. Evaluation of dietary energy intake and physical activity in dogs undergoing a controlled weight-loss programe. J Am Vet Med Assoc 2012, 240(4): 413-419.



