> Abstract
Peripheral nerve damage can occur as a consequence of accidental or iatrogenic injury caused by sharp or blunt trauma. Damage to peripheral nerves often accompanies orthopedic injuries (e.g. fracture, dislocation) and of particular clinical importance is considered the damage to nerves of the limbs. The mechanism of nerve degeneration and regeneration after nerve injury is complex. Failure of nerve restoration of normal function and the emergence of complications may both lead to permanent disability. Knowledge of pathophysiology and regeneration process is considered fundamental for the clinician, because clinical symptoms can be interpreted more accurately. Serial physical examinations and electrophysiology studies can be used to evaluate the prognosis and the suitable method of treatment. In many cases surgical exploration of the affected area can provide accurate prognostic information. Regardless of the treatment method chosen, recovery progresses relatively slow. The owner should be informed about the increased nursing care requirements involved in those cases, because his collaboration is considered essential in their management.
> Introduction
Traumatic nerve injuries are quite common in small animal practice and they are observed in animals of any age. Of great clinical importance is the damage of nerve branches dispersing to the fore- and hind limbs, which commonly require limb salvage surgery. Comprehension of the mechanism of nerve injury and repair guides the practitioner through the decision making process, in order permanent nerve damage to be avoided.
> Anatomy
Neuron is the primary anatomical unit of the nervous system (Figure 1). A typical neuron has a long shape with multiple cell processes, which according to the direction in which the electrical impulse is transmitted, they are distinguished in axons and dendrites. Dendrites are usually multiple and carry electrical signals toward the cell body, while the nerve cell branch called axon single and carries an impulse away from the cell body. Axons can be classified as unmyelinated and myelinated. The latter ones are covered by a fatty coating known as myelin sheath which in the peripheral nervous system is formed by the cells of Schwann, which surround the axon (Schwann’s membrane or neurilemma). The junctional areas, located at discrete intervals between Schwann cells, are called nodes of Ranvier and they allow for more rapid conduction of impulses. On the contrary, the transport of impulses in unmyelinated axons is accomplished with lower speed, because the electric potential is interspersed in their whole length 1, 2.
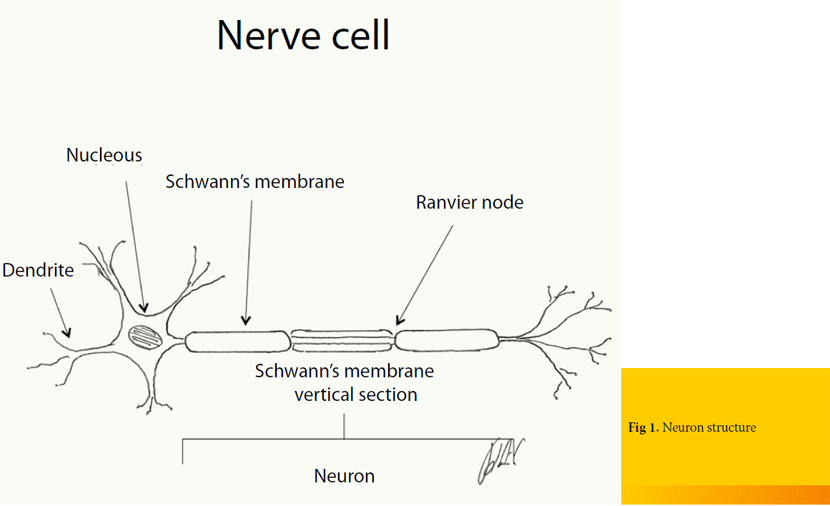
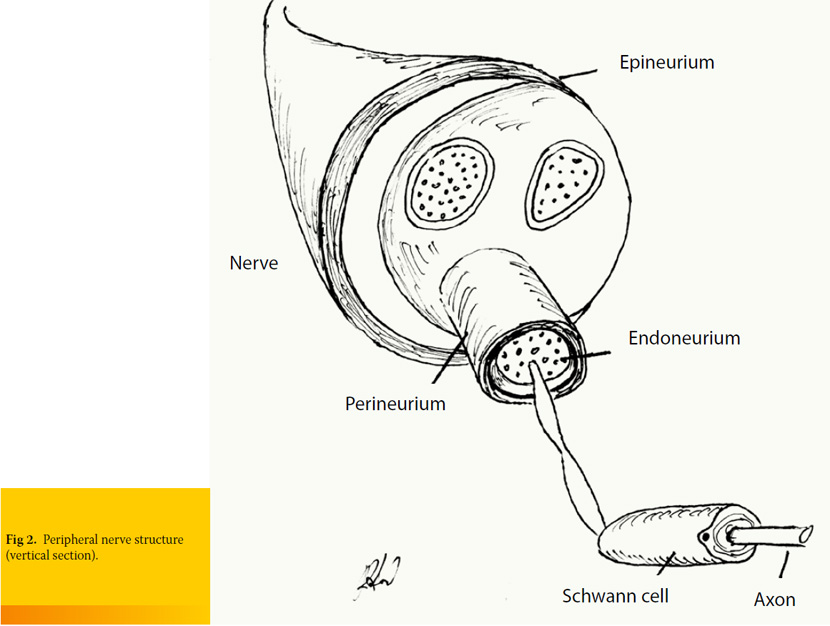
Each axon (nerve fiber) is covered by connective tissue known as endoneurium. This is loose connective tissue which is composed of collagen, fibroblasts and a capillary system that provides nourishment to each nerve fiber. Axons are organized in bundles of various thicknesses. Each bundle is surrounded by perineurium which is a tough fibrous sleeve composed of collagen and elastic fibers that lends protection and structural support. Nerve is formed by various bundles of axons, surrounded by perineurium, which is consisted of collagen fibers of looser organization, compared to perineurium and endoneurium.1, 2 (Figure 2).
> Classification of nerve damage
Peripheral nerve injuries have been classified into three categories: neurapraxia, axonotmesis, and neurotmesis.1 Neurapraxia is a transient conduction block of the physiological nerve transmission, usually associated with a lesion of myelin around the axons, that will typically resolve by 3 to 6 weeks.3 Axonotmesis refers to the disruption of axons while the internal architecture of the nerve is maintained intact. Spontaneous recovery does occur, although not as rapidly as with neurapraxia. Furthermore, neurotmesis is called the complete separation of the nerve. Spontaneous recovery rarely occurs, because of separation and misalignment of the nerve stumps. Proliferation of fibrous tissue and Schwann cells and axonal growth result in loss of nerve architecture and function 4
Peripheral nerve damages have also been classified according to their degree of severity. First-degree damage causes generation and conduction blockage at the site of injury with all the components of the nerve being intact. Second-degree damage is characterized by axonal disruption and Wallerian degeneration, while third-degree damage is accompanied by disruption of the axon and its related endoneurial tube. In fourth-degree damage, epineurium is the only structure that remains intact. Fifth-degree damage is the complete severance of all the nerve s structures.5
> Aetiology
Acute peripheral nerve injury in small animals most often occurs as a result of trauma.3 Reported common types of trauma include automobile accidents, 6 gunshots, bite wounds, lacerations and iatrogenic damage during surgical procedures, application of splints or casts and injection of therapeutic agents.2
Traumatic peripheral neuropathies often accompany orthopaedic injuries (e.g. fracture, dislocation) involving the pelvis, the shoulder and the long bones. Sciatic nerve injury is a reported common complication of pelvic fractures and the surgical manipulation of the hip joint.7-10 According to a retrospective study, iliac body fractures are considered the most common cause of partial or complete paralysis of the sciatic nerve in dogs.8 Furthermore, fractures of the distal humerus are among the common causes of radial nerve injury. 11
Peripheral nerve injuries can be classified into open and closed. Open lesions are usually caused by penetrating injuries and they are often accompanied by extensive damage and inflammation of the surrounding tissues. In contrast, closed nerve lesions may result from crushing, contusing, or stretching injuries.3 Extreme abduction or caudal traction of a limb usually results to avulsion of the nerve roots. Avulsion typically occurs at the level of the intradural dorsal and ventral spinal roots and leads to Wallerian degeneration.12 The roots, due to the lack of perineurium, are rendered less elastic, and thus more vulnerable to traction compared to peripheral nerves.13 Perhaps, this explains why the avulsion of the nerve roots of the brachial plexus is the most common neurological condition of the forelimb.14 It typically occurs after extreme abduction of the shoulder or traction of the forelimb.15According to studies performed in cats, ascertaining the amount of stretch that a nerve can withstand before damage occurs, it was demonstrated that their peroneal nerves may be stretched 100 percent of their original length and yet functional recovery is still complete 14 days after stretching. 2
> Pathophysiology
Nerve damage is followed by hemorrhage and projection of a blood clot from the cut nerve ends. Accumulation of blood, plasma and hydrophilic acid mucopolysaccharide, leads to local swelling at the area of damage. Swelling persists for approximately a week and then it slowly subsides.16
According to the theory of Wallerian degeneration, the severance of an axon is immediately followed by degeneration, initially of the proximal stump and then of the distal stump. Histologically, the degeneration is similar in both nerve ends, but in the proximal stump it is usually limited to one to three nodes of Ranvier.17 The proximal extent of the traumatic degeneration varies according to the extent of the inciting injury and greater damage is expected after avulsion or crushing injuries rather than sharp laceration.18 The axons at the proximal end of the peripheral nerve stump tend to enlarge and isolate themselves and survive for approximately 2 weeks.16 At the distal end of the peripheral nerve stump occurs complete degradation of the axons by 48 to 96 hours.19 One week post injury, no anatomical structures are recognized in affected area. Connective tissue and Schwann cell debris are left instead. In the early stages, degeneration starts from the outer layer soft myelin, due to intense enzymatic activity of the surrounding Schwann cells.2 Degeneration of the axons and myelin gives rise to breakdown products which are removed by macrophages and Schwann cells.2,16 Macrophages originate from histiocytes present in the connective tissue and the blood vessels of the nerve.2 Neuromuscular junctions are also involved in the degenerative process, as motor endplates disappear 3-5 days after nerve severance. It has also been reported that synapses degenerate simultaneously or even prior to degeneration of the axon. However, experimental studies in dogs have shown that neuromuscular junctions remain functional for approximately five days following nerve severance.20
Proliferation of connective tissue, Schwann cells and capillaries begins concurrently with the onset of increased metabolic activity of the neuron. 1 to 3 days after the injury, fibroblasts of epineurial origin, endoneurial connective tissue within the nerve, and capillaries begin to proliferate at the proximal nerve stump. Regenerating axons will grow along this bridge of healthy tissue, to reach the distal stump. These axons sprout from the proximal nerve stump 4 to 20 days after their injury and advance toward the distal nerve stump in an average axonal growth of 1-2mm per day. This process is the same for both sensory and motor nerves. In cases of traumatic nerve severance axonal budding begins 1 to 3 cm proximal to the point of severance. However, after laceration by sharp object nerve, axonal regeneration begins a few millimeters proximal to the last intact node of Ranvier.2
As soon as the second day, Schwann cells of the two nerve stumps proliferate and tend to form longitudinal columns, called “bands of Bungner”.21 Those bands are continuous with the persisting Schwann tubes in each nerve stump and serve as a guiding mechanism for the regenerating axons. Once an axon has penetrate a Schwann tube, remyelination begins with myelin production by Schwann cells.2
In case that the nerve ends do not approximate to each other, they become increasingly contracted and replaced by connective tissue.3 Even if anatomical contact is eventually carried out, the tubes may not exhibit adequate length to cover the axon segments by the appropriate amount of myelin.17 Likewise, some motor axons may enter neurilemmal tubes of sensoryneurons and vice versa, rendering them nonfunctional.2, 17
When a motor axon is being transected, the denervated muscle gradually undergoes atrophy. If the regenerating motor nerve axon manages to reach the muscle, he will selectively reinnervate old endplate sites.19 This reinnervation further ignites the regeneration of the axon.17 It has been stated that in the proximal muscle groups of humans, the maximum time tolerated between the time of nerve injury and the reestablishment of neuromuscular connection is between 1 year and 18 months.18 During small animal patients’ nerve regeneration muscle atrophy, permanent muscle contracture and/or self-amputation of the affected limb may occur, rendering a guarded prognosis. Further, the longer the receptor organ remains without innervation, the more nerve degeneration proceeds, and it is likely that the reinnervated sensory receptors are not functional anymore.17
Despite the fact that neuromas of the proximal stump and schwannoma or glioma of the distal stump have been consistently observed in experimenta studies,22 they are considered of little clinical importance in dogs.23 Neuromas and gliomas are formed by the organization of thrombi at the ends of the nerve stumps, which is followed by the randomly oriented growth of Schwann cells and axons and remyelination of the above formation. Schwannoma is formed in the same way as a neuroma. However, axonsare not involved in the formation of schwannoma.16
Average axonal regeneration is at a rate of 2.5 cm per month and the majority of nerves in dogs rarely exceed 50 cm in length. For this reason, the prognosis for return to function is good if the distance between the site of injury and the end plateis less than 10 to 15 cm, and guarded if the distance from the end plate is greater than 25 to 30 cm.17 Experimental studies have shown that axonal growth occurs at a rate of 3 to 4 mm per day, but the rate of functional recovery is not more than 1 to 2 mm per day. The degree of functional recovery varies from 80% of normal in motor nerves to 50% or 60% of normal in mixed nerves.16
> Diagnosis
At admission, animals usually suffer from concurrent orthopaedic problems, which can overshadow the neurological symptoms and mislead the clinician, unless he performs a complete and careful clinical examination.
Peripheral nerve damage is manifested as diminished or absent sensation and/or motor function, which is assessed by examining the spinal reflexes. Reflexes in the triceps and biceps of the forelimbs are unreliable.24 In the hind limbs, the quadriceps, cranial tibial and sciatic reflexes are evaluated. Withdrawal (flexor) reflexes should be examined in all limbs. The response involves all of the flexor muscles of the limb and thus requires the activation of motor neurons in several spinal cord segments.24
Moreover, deep pain sensation is assessed by observing the animal for signs of conscious perception of the stimulus created by grasping a small amount of skin, lifting it slightly and pinching it with hemostats.24 An autogenous zone is the cutaneous region that receives sensory innervation from only one peripheral nerve.15 Diminished or absent sensation in an autogenous zone reflects damage to that specific nerve. Finally, motor function is further assessed by evaluating the muscle tone of the corresponding muscles .25
Assessment of sensory and motor nerve function is followed by evaluation of the extent of damage. This can be achieved indirectly, combining findings from the physical examination and the degree of functional improvement during the follow up period, or directly with the aid of electrodiagnostic examinations. The animal’s examinations should be repeated at a regular basis for 8 weeks. This time period may be longer in case of improvement evidenced by physical or laboratory examination.14
Avulsion of the caudal brachial plexus roots (C8- T2) results in carriage of the limb with the elbow and shoulder flexed. Flexion is possible because musculocutaneous, axillary, and suprascapular nerves are preserved. In contrast, in damage to C7-T1spinal cord segments, the elbow appears dropped because of the loss of function of the majority of shoulder extensors. A limb that is knuckled onto digits is observed in both instances (Figure 3). Cutaneous analgesia, as it was mentioned above, includes the autogenous zone of the injured nerve. Ipsilateral miosis, absence of a cutaneous trunci reflex, and neurogenic atrophy are additional clinical signs of brachial plexus injury. Miosis occurs as part of Horner’s syndrome caused by trauma to the T1 segment and T1 and T2 ventral nerve roots, which results in interruption of sympathetic innervation to the ipsilateral eye. The fully developed Horner’s syndrome (miosis, upper eyelid ptosis, enophthalmos, and nictating membrane protrusion, conjuctival hyperemia) is not usually seen in dogs.14
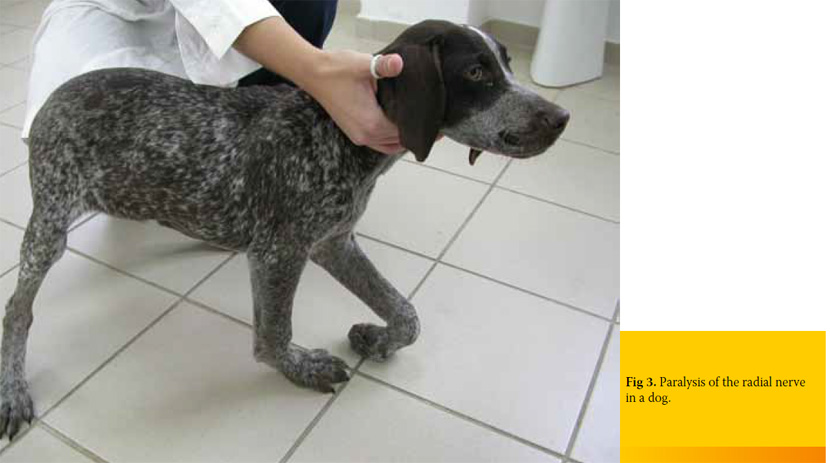
In animals with sciatic nerve paralysis the clinical signs are a dropped hock and knuckling of the digits. The limb can support weight because the quadriceps muscle is functional due to the integrity of the femoral nerve.26 Sensory analgesia is detected on the lateral, dorsal and plantar surfaces of the lower limb. The medial side of the limb is innervated by the saphenous nerve, a branch of the femoral nerve, and will exhibit sensation. The cranial tibial and sciatic reflexes are absent, as is the withdrawal reflex. In case of peroneal nerve paralysis hyperextension of the tarsus and knuckling of the digits are observed (Figure 4). Cutaneous analgesia is also present on the dorsal and cranial aspect of the limb, distal to the stifle. In contrast, clinical signs of tibial nerve injury include a dropped hock and overextended digits with analgesia of the caudal/plantar surface of metatarsus f and digits.8

Femoral nerve damage is not common, because the femoral nerve and its roots are covered in their origin by the lumbar fascia. However, when present, it causes severe posture and gait disturbances. Clinical signs include a limb unable to bear weight as the stifle joint is permanently flexed and the hip range of flexion is decreased. Patellar tendon reflex is decreased or absent and atrophy of quadriceps muscle is often observed. Sensation affects the inner surface of the hind limb and the first phalanx as well.11
Electrophysiological testing is a substantial method for evaluating the function of muscles, neuromuscular junctions, peripheral nerves and central nervous system. It is reported that electrophysiological assessment should be performed 5 to 14 days after the initial trauma,1 because nerve function is decreased or absent immediately after the injury, albeit the peripheral part of the axon maintains its ability to conduct the electrical activity for 72 hours.17 Those tests estimate the action potentials of the muscle during rest and contraction and the potentials generated by the insertion of the needle into the muscle. When indications of nerve dysfunction are present, nerve stimulation tests should be performed instead, as they are more specific concerning their ability to confirm nerve’s integrity.27
Physical examination and electromyography should be repeated regularly in order to evaluate the extent of damage. If electrodiagnostic testing is not available, the repetition of neurological examination every 2-4 weeks for 2-3 months is highly recommended.17
It is reported that during the exploratory surgery of an injured nerve, its appearance and vitality witness the type of damage. Generally, normal gross appearance of a nerve indicates axonotmesis or neurapraxia. A fusiform neuroma usually indicates severe axonotmesis or intraneural hematoma. The texture of the neuroma has been associated with prognosis. A fluctuant neuroma is associated with better outcome, compared to a hard neuroma, which is indicative of a poor prognosis. Bulbous neuromas usually indicate neurotmesis and poor regeneration capacity, while lateral neuromas indicate partial neurotmesis. Partial neurotmesis that does not exceed ½-¾ of the nerve’s width does not require surgical repair.28 Moreover, a dumbbell-shaped neuroma suggests that the nerve has been completely transected and the ends are held in approximation only by scar tissue. However, palpation of neuromas can be misleading, and it should be used only as ancillary diagnostic method.29
When exploratory surgery is undertaken, an incision is made perpendicular to the lesion and the latter is scrutinized for healthy nerve fibers. If the incision exceeds ½-¾ of the original diameter of the nerve, complete resection and anastomosis is recommended. Another method has been described in order to evaluate the degree of injured nerve fibrosis using intraneural injection of saline solution tinted with a harmless dye. If the liquid spreads freely between the funi culi and beneath the sheath, scar tissue is not present. 29
> Prognosis - General therapeutic plan
As it has been mentioned above, nerve regeneration is influenced primarily by the extent of damage of the surrounding supporting structures and the distance between the point of lesion of the nerve and the cell body or the end organ. The integrity of endoneurium and Schwann cells, during axonotmesis and neurapraxia ensure better outcome, contrary to neurotmesis, in which the proliferation of scar tissue may impede the process of axon regeneration.30
The prognosis of brachial plexus injuries is guarded to poor and the possibilities for nerve repair are low.31 In contrast, sciatic nerve injury, even the iatrogenic one, has been associated with an excellent prognosis for recovery.8 The presence of open wounds across the nerve course or iatrogenic trauma to the nerve, constitutes clear indication for immediate surgical investigation and treatment.8, 18
It is suggested that blunt nerve injuries should be treated two to three weeks following injury, especially if they are located to the central part of the nerve. On the contrary, sharp lacerations of nerves located more distally are best treated on the day of injury.2 In cases of simple lacerations, immediate anastomosis of the nerve ends is recommended, provided the proper equipment and expertise are available. Otherwise, incompetent attempts to perform an anastomosis may lead to inflammation and fibrosis, that complicate the regeneration process.4 If the proximal and distal stump of the injured nerve cannot be accurately assessed at the time of initial exploration or if extensive fibrous connective tissue formation or infection is found, the nerve ends are tacked down to adjacent muscle to prevent retraction. A second surgical intervention is performed in approximately 3 weeks.18 Delayed treatment is advantageous because adjacent tissue inflammation has resolved, axonal sprouting has already commenced and epineurium is thinner, less swollen and better able to hold sutures.3
In the majority of nerve injuries, anastomosis is accomplished with 4-6 simple epineurial sutures, particularly when the loss of nervous tissue is minimal.4 The nerve ends are isolated from the surrounding tissues and debrided.1 Sutures must not penetrate perineurium or endoneurium and the repair must be tension free.1 Anastomosis is performed with 8-0 to 10-0 monofilament non absorbable nylon suture and an operating microscope is occasionally required.4
It has been reported that external or internal neurolysis may be performed on a damaged nerve instead of resection and anastomosis. External neurolysis is performed by freeing the nerve from the surrounding scar tissue and placing it in a bed free of scar tissue. Internal neurolysis involves incising the epineurium longitudinally in the area of involvement and beyond, and separating the nerve fibers.25 Data from previously performed electrodiagnostic tests are helpful regarding the choice of the suitable technique, while the presence of function constitutes criterion for the application of neurolysis.2
Closed nerve damage should be managed conservatively, by bandaging the distal portion of the limb in order to prevent trauma and by physical therapy, in order to prevent muscle atrophy and fibrosis. Neurogenic atrophy may be seen faster than any other kind of muscle atrophy, even in 10- 14 days after the initial trauma.32 Physical therapy should be continued during the recovery period, until clinical signs have beensubsided.17
In spinal nerve injury, amputation of the affected limb is considered if total analgesia is present distal to the elbow and stifle joint and/or repeated attempts of self-injury occur.32However clinical improvement is still possible, thus it is recommended to delay amputation for up to 6 months.17
Apart from amputation, several methods of management have been described for monoparesis, especially when nerve damage is partial and some function is still preserved. Arthrodesis of the distal joints, transposition of tendons and nerve transplantation has been reported. The surgeon should decide whether these procedures are necessary because their reported results vary.15, 33-36
The role of the owner in the management of such patients is quite important. Performance of appropriate and repeated physiotherapy (3-4 times daily), relies heavily on his consistency and patience. Supporting the animal and preventing its self- injuries during the rehabilitation period are also responsibilities of the owner. Amputation is often disapproved, because it is assumed that it will affect the welfare of the animal. Thus, owners should be thoroughly informed by the clinician about their animal’s neurological status and the possible clinical outcome.
> Conclusion
In patients with post-traumatic disturbances of the gait, meticulous examination of the neuromuscular system should not be omitted. Even if, in general terms, the prognosis of peripheral nerve injury is guarded, every effort should be done for the reestablishment of nerve function. The surgeon, in this effort, should have active support by a dedicated owner. Moreover, extended follow up is important in those cases and its significance must be underlined to the owner.
> REFERENCES
1. Welch JA. Peripheral nerve injury. Semin Vet Med Surg (Small Anim) 1996, 11: 273-284.
2. Swaim SF. Peripheral neuropathies. Ιn: Pathophysiology in Small Animal Surgery. Bojrab MJ (ed). Lea & Febiger: Philadelphia, 1981, pp. 233-242.
3. Swaim SF. Peripheral Nerve Surgery. In: Veterinary Neurology. Oliver JE, Hoerlein BF, Mayhew IG (eds). Saunders: Philadelphia, 1987, pp. 493-512.
4. Rodkey WG. Peripheral nerve surgery. In: Disease Mechanisms in Small Animal Surgery. Bojrab MJ (ed). Lea & Febiger: Philadelphia, 1993, pp. 1135-1141.
5. Sunderland S. Nerves and Nerve Injuries. Williams &. Wilkins: Baltimore, 1968, pp. 808-885.
6. Knect CD, St. Clair LE. The radial-brachial paralysis syndrome in the dog. J Am Vet Med Assoc 1969, 54: 653-656.
7. Fanton JW, Blass CE, Withrow SJ. Sciatic nerve injury as a complication of intramedullary pin fixation of femoral fractures. J Am Anim Hosp Assoc 1983, 19: 687-694.
8. Gilmore DR. Sciatic nerve injury in twenty-nine dogs. J Am Anim Hosp Assoc 1984, 20: 403-407.
9. Chambers JN, Hardie EM. Localization and management of sciatic nerve injury due to ischial or acetabular fracture. J Am Anim Hosp Assoc 1986, 22: 539-544.
10. Kuntz CA, Waldron D, Martin RA, Shires PK, Moon M, Shell L. Sacral fractures in dogs: A review of 32 cases. J Am Anim Hosp Assoc 1995, 31: 142-150.
11. Anor S. Monoparesis. In: BSAVA Manual of Canine and Feline Neurology. Platt SR, Olby NJ (eds). 3rd edn. BSAVA, Gloucestershire, 2004, pp. 265-279.
12. Griffiths IR. Avulsion of the brachial plexus-1. Neuropathology of the spinal cord and peripheral nerves. J Small Anim Pract 1974, 15: 165-176.
13. Shores A. Traumatic and neoplastic diseases of the brachial plexus. In: Disease Mechanisms in Small Animal Surgery. Bojrab MJ (ed). Lea & Febiger: Philadelphia, 1993, pp. 1175-1182.
14. Griffiths IR, Duncan ID, Lawson DD. Avulsion of the brachial plexus-2. Clinical aspects. J Small Anim Pract 1974, 15: 177-182.
15. Steinberg HS. Brachial plexus injuries and dysfunctions. Vet Clin N Am-Small 1988, 18: 565-580.
16. Ducker TB. Metabolic factors in surgery of peripheral nerves. Surg Clin North Am 1972, 52: 1109-1122.
17. Crisman CL. Peripheral Neuropathies. In: Disease Mechanisms in Small Animal Surgery. Bojrab MJ (ed). Lea & Febiger: Philadelphia, 1993, pp. 1158-1173.
18. Mackinnon SE, Dellon AL. Nerve repair and nerve grafting. In: Surgery of the Peripheral Nerve. Mackinnon SE, Dellon AL (eds). Thieme: New York, 1988, pp. 89-129.
19. Seckel BR. Enhancement of peripheral nerve regeneration. Muscle & Nerve 1990, 13: 785-800.
20. Sims MH, Redding RW. Failure of neuromuscular transmission after complete nerve section in the dog. Am J Vet Res 1979, 40: 931-935.
21. Asbury AK, Johnson PC. Pathology of peripheral nerves. In: Major Problems in Pathology, 9. Bennington JL (ed). WB Saunders: Philadelphia, 1979, pp. 148.
22. Robins G. Dropped jaw-mandibular neurapraxia in the dog. J Small Anim Pract 1976, 17: 753-758.
23. Hoerlein BF. Canine Neurology: Diagnosis and Treatment. 3rd edn. WB Saunders: Philadelphia, 1978, pp. 470-560.
24. Oliver JE, Lorenz MD. Neurologic examination. In: Handbook of Veterinary Neurologic Diagnosis. Oliver JE, Lorenz MD (eds). Saunders: Philadelphia 1993, pp.19-57.
25. Lorenz MD, Michael D. Confirming diagnosis. In: Handbook of Veterinary Neurology. 5th edn. Lorenz MD, Coates JR, Kent M (eds). Saunders: Philadelphia, 2011, pp. 75- 92.
26. Chambers JN, Hardie EM. Localization and management of sciatic nerve injury due to ischial or acetabular fracture. J Am Anim Hosp Assoc 1986, 22: 539-544.
27. Lorenz MD, Michael D (2011). Paresis of one limb. In: Handbook of Veterinary Neurology. 5th edn. Lorenz MD, Coates JR, Kent M (eds). Saunders: Philadelphia, pp. 94-108.
28. Rizzoli HV. Treatment of peripheral nerve injuries. In: Neurological Surgery of Trauma. Coates JB, Meirowsky AM (eds). US Gov Printing Office: Washington, 1965, pp. 565.
29. Smith JW. Microsurgery of peripheral nerves. Plast Reconstr Surg 1964, 33: 317-329.
30. Midgley RD, Woolhouse FM. Silicone rubber sheathing as an adjunct to neural anastomosis. Surg Clin North Am 1968, 48: 1149-1157.
31. Steinberg S. The use of electrodiagnostic techniques inevaluating traumatic brachial plexus root injuries. J Am Anim Hosp Assoc 1979, 15: 621-626.
32. Wheeler SJ, Jones DG, Wright JA. The diagnosis of brachial plexus disorders in dogs: A review of 22 cases. J Small Anim Pract 1986, 27: 147-157.
33. Hussain S, Pettit GD. Tendon transplantation to compensate for radial nerve paralysis in the dog. Am J Vet Res 1967, 28: 335-44.
34. Lorinson D, Groblinger K. Tendon transfer after brachial plexus injury in 4 dogs and 2 cats. Proceedings of the 1st World Orthopaedic Veterinary Congress: Munich, 2002, pp. 135.
35. Bennett D, Vaughan LG. The use of muscle relocation techniques in the treatment of peripheral nerve injuries in dogs and cats. J Small Anim Pract 1967, 17: 99-108.
36. Sterner W, Moller AW. Tendon transplantation – A surgical approach to radial paralysis in the dog. J Am Vet Med Assoc 1960, 137: 71-75.



