Improving by understanding
> Abstract
Feline chronic kidney disease (CKD) is characterized by irreversible structural lesions of the kidneys and may lead to chronic renal failure (CRF), which eventually results in accumulation of metabolic toxins and dysregulation of fluid, electrolyte, and acid-base balance. CKD mainly affects geriatric cats. In the majority of animals the initiating factor of CKD remains unclear. Idiopathic, familial, congenital, inflammatory, infectious, and neoplastic causes have been suggested. Once lesions have adequately progressed, the condition is generally self-perpetuated. The main clinical signs are anorexia, weight loss, vomiting, and diarrhoea. Early diagnosis of CKD is crucial. Anaemia, azotaemia, hyperphosphataemia, and hypokalaemia may be detected by laboratory examination. Radiology and ultrasonography of the abdominal cavity may contribute to identification of the initiating factor. Renal histopathology may aid in diagnosing the primary cause. Consequences of CKD are multisystemic and include arterial hypertension, renal secondary hyperparathyroidism, anaemia, gastrointestinal complications, and acid-base and diverse electrolyte disturbances.
> Introduction
Chronic kidney disease (CKD), which may result in chronic renal insufficiency (CRI) or failure (CRF), is very common among the feline population and is one of the main causes of death in elderly patients.1-3 Kidney injury may occur at any time during a cat’s life, but it may not be evident until CRF becomes apparent. CRF is characterized by irreversible kidney damage, resulting in compromised structural and functional integrity of the kidney. The kidney damage leads to dysregulation of body fluids and acid-base balance, and to decreased removal of metabolic waste products. It may also result in eliminating of production of various hormones or being the target organ for them such as errhythropoeitin, 1,25-dihydroxycholocalsipherol (calcitriol), antidiuretic hormone, renin, and aldosterone.3,4 Eventually, the clinical manifestation of advanced kidney failure is uraemia.5 Taking into account the severity of serum creatinine concentration, proteinuria, and arterial hypertension, the International Renal Interest Society (IRIS) has developed a staging system for CKD, which is characterized by four stages.2
> Aetiology
Feline CKD may be caused by various factors that directly or indirectly have an effect on the kidneys. Irrespective of its cause, when renal damage is pursued, CKD is considered a progressively deteriorating disorder. Renal insufficiency becomes apparent when more than 3/4 (75%) of the kidneys are damaged. Signs of uraemia are usually present when lesions extend to 80-85% of the kidneys.6,7
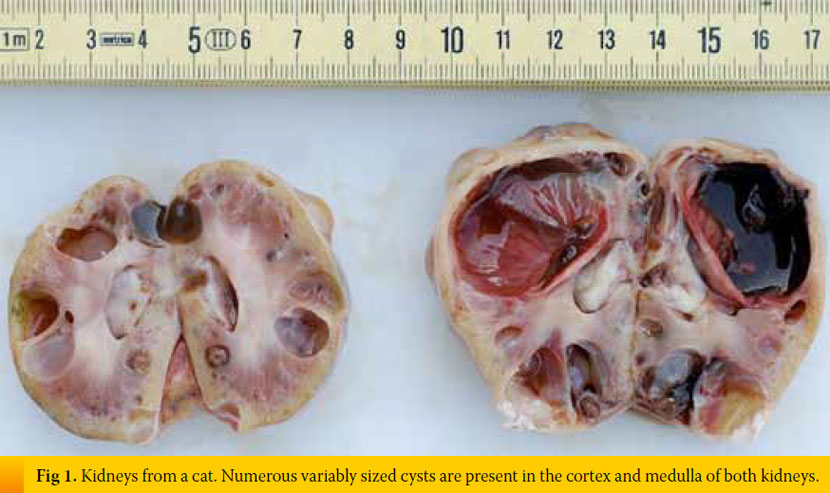
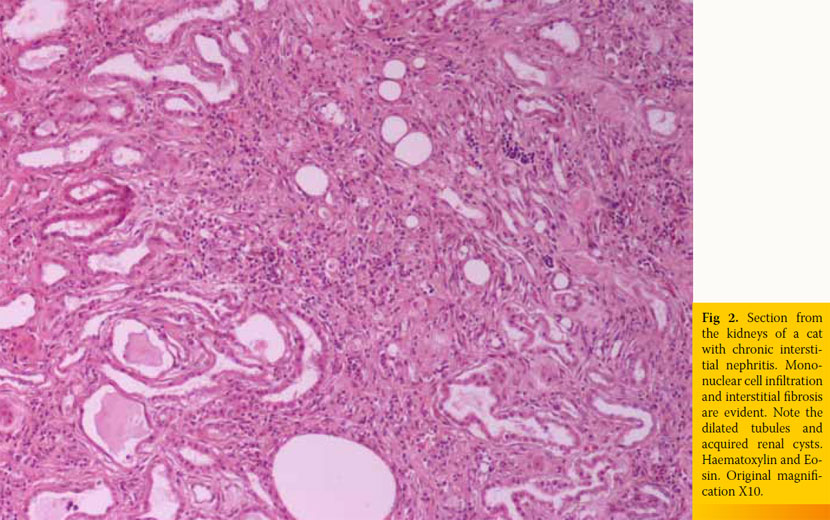
Inherent, congenital, inflammatory, infectious, or neoplastic factors have been suggested as causes of CKD,2,3 although the causative agent in approximately 50% of feline cases was idiopathic interstitial nephritis (Table 1).8 Recently, Lawler et al1 suggested that feline CKD may be part of a normal aging phenomenon and a survival driven adaptive process. Moreover, polycystic kidney disease is common in Persian cats (Fig 1). Its overall prevalence varies geographically for this breed and reaches almost 50% in Great Britain and France.9,10 Although there are no published studies demonstrating the aetiopathogenetic relationship between CKD and nephrolithiasis, Ross et al11 recently found that nephrolithiasis did not positively correlate with progression of CKD. Some cases of CRF in cats were attributed by Minkus et al12 to immune mediated mechanisms, due to infiltration of the kidneys by lymphocytes and plasmacytes, suggesting chronic tubulointerstitial nephritis (Fig 2). Furthermore, Lappin et al13 was not able to correlate vaccination of cats against herpesvirus (FHV), calicivirus (FCV), and panleukopenia (FPV) with CKD. Recurrent or untreated episodes of feline lower urinary tract disease,14 a very common clinical entity in cats, and infection by feline immunodeficiency virus may eventually result in CRF. German et al15 suggested that feline foamy virus, which is classified as a retrovirus and is considered quite widespread among cat populations, could have been the cause of glomerulonephritis detected in experimentally infected cats.
An interesting disorder occurs when the ureter is obstructed due to urolith, ureteritis, or from accumulation of debris, and the glomerular filtration rate (GFR) in the other kidney is reduced due to CKD. This entity is an “acute over chronic” renal failure. Cats present with uraemic crisis due to lesions of CKD and subsequent malfunction of the other kidney. Upon abdominal palpation a small and irregular kidney is evident, while the other is swollen and perhaps painful. This entity has been called “big kidney – little kidney syndrome”.16
It has been suggested that the inciting cause affects initially the tubolointerstitium,7,17 but it is currently believed to be elusive. However, lesions that are confined to a specific anatomical component of the kidney progressively extend to the remaining.4,7,17 In most cats with CRF, the causative agent may not be detected despite proper diagnostic evaluation. This was confirmed by various studies where even after kidney histopathology, a definitive diagnosis was not established,12,18 and it may be attributed to the functional independence of various components of nephrons, the limitation of responses of various kidney parts to different agents and the inability of the nephrons to be replaced when irreversibly destroyed. In one study, chronic tubulointerstitial nephritis was detected in 70.4% of cats with CRF, while membranous glomerulonephritis, neoplasia (specifically lymphoma), and amyloidosis were observed in 14.8%, 11%, and 2% of cats with CRF, respectively.12
> Pathogenesis
In humans and dogs, CKD is characterized by progressive regression of kidney function; while in cats a stepwise deterioration of GFR may be noticed, suggesting uraemic crisis may be detected suddenly in an otherwise stable patient.6,19 During CKD, many nephrons become non-functioning. Viable nephrons hyperfunction and become hypertrophied and hyperplastic to complete the work of the kidneys. Moreover, intraglomerular hypertension is evident, leading to these nephrons being damaged rapidly. Although this hyperfiltrative state was formerly considered beneficial, it is currently believed to be detrimental.20,21 Renal function deterioration is expected sooner in tubulointerstitial nephritis than in glomerular disease,20 although life expectancy was longer in cats with tubulointerstitial nephritis compared to those with other nephropathies.1 Additionally, proteinuria due to glomerulopathy has been suggested to contribute to intrinsic renal toxicity and further nephron damage, but the precise role of proteinuria in the progression of renal disease is uncertain.22 Although not confirmed in cats, nephron damage from proteinuria has attributed to tubular cell death due to albumin reabsorption, the toxicity of transferin carrying iron, or the effect of various cytokines.20 It is still controversial whether proteinuria is a marker or a mediator of feline CKD.22 Nevertheless, if the inciting cause still exists, further deterioration of renal function is expected2,20 leading to substantial renal damage.23 Finally, the consequences of CRF have a direct or indirect impact on the kidneys themselves.24
> Diagnostic evaluation
Diagnostic evaluation of feline CKD should aim to the identification, if possible, of the cause of kidney disease, and to the estimation of its severity. Furthermore, it should determine its consequences, investigate any concurrent conditions and establish the rate of loss of kidney function.
Signalment
CRF usually presents in middle aged to elderly cats,2,25,26 although young cats may be also affected with 10-15% of them younger than 3 years old.8 Evaluation of renal function is considered imperative in older cats because CKD affects up to 31% or 32% of cats older than 10 or 15 years, respectively.2 In general, CKD presents in 1.6%27 to 20% of cats examined for various reasons.28
No sex predilection has been reported, although a slight increase in males has been found.26 Recently, White et al29 reported that male cats exhibited CKD in a younger age than females. The influence of sex hormones, evident in humans, could be a rational explanation, but this hypothesis comes in contrast with the usually neutered patients of the reference population of veterinary practices.29 Maine coon, Abyssinian, Siamese, Russian blue, Persian, Angora, and Burmese cats are overrepresented.2 However, this depends on the study population; in our clinic and probably others too,18 Siamese and domestic shorthaired cats are most commonly presented. Familial predisposition to kidney disease resulting in CRF is evident in certain breeds, such as polycystic kidney disease in Persians, and amyloidosis in Abyssinians and possibly Ragdoll cats.30
Clinical signs
Cats may present with various signs; their severity appears to vary directly with the development of uraemia. Owners usually seek medical help when clinical signs are profound, suggesting advanced disease. However, the cat may not present initially with any clinical signs or it may show polyuria/polydipsia persisting for weeks or months (1st stage of CKD). Polyuria/polydipsia, which is a rather constant finding in dogs with CRF, was observed in only 40% of cats with CRF in one study.18 Moreover, decreased appetite, weight loss, and poor hair coat may be seen at the initial stage; whereas in advanced stages, more intense clinical signs (uraemia) are evident including sporadic (at least initially) vomiting, diarrhoea, and ocular manifestations.8 Vomiting is not a common sign in cats with CRF, perhaps due to a low frequency of gastritis.18 Although rare, blindness may be the only sign recognized by cat owners.6,31
Physical examination
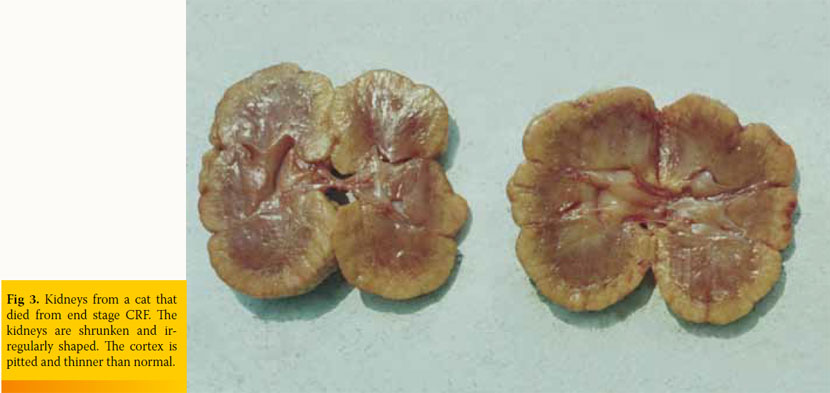
Physical examination findings in cats with CKD are generally nonspecific. Depending on the stage of the disease the cat may present with dehydration and weight loss, poor haircoat, depression, pale mucous membranes, oral ulceration, halitosis, drooling, weak pulses, dyspnoea, and hypothermia.6,8 Upon abdominal palpation, small-sized, irregular, and painless kidneys may be evident, which in the authors’ experience is rather an occasional finding in cats with spontaneous CKD (Fig 3). Renomegaly may also occur and, more commonly, normal renal size is reported.18 Cardiac arrhythmias and heart murmur, as well as increased breathing sounds or crackles, may be found on auscultation. Ocular manifestation includes hyphaema or oedema, haemorrhage, detachment, and vessel tortuosity of the retina.31
Laboratory evaluation
Early recognition of CKD in cats is crucial. In its early stages, therapeutic intervention may delay or prevent its progression.4,7 Diagnostic evaluation of kidney disease is mainly based on laboratory findings because history and physical examination is usually non-specific. Of course, polyuria/polydipsia of long duration and/or small sized kidneys (single or both) on palpation may lead to serious suspicions of CRF.6
Various haematological and biochemical findings reported in cats with CRF are listed in Table 2. Cats with CRF may present with mild to moderate normochromic and normocytic non-regenerative anaemia.2 Leukocytosis is usually secondary to the inciting cause. Azotaemia is the main biochemical abnormality, which is characterized by increased concentration of serum urea and creatinine. However, it has been reported that 60% of cats with CRF have increased concentrations of phosphorus, and 20-30% of them have decreased concentrations of potassium,31 whereas potassium is increased in 15% of cases.6 The concentration of calcium is either increased, decreased, or normal.8 The concentrations of magnesium and sodium are decreased6,25 or usually normal. The concentration of chloride is decreased mainly when metabolic acidosis is present, which often accompanies the advanced stage of the disease (4th stage of CKD).24 It seems that acidosis is more commonly observed in cats in uraemic crisis,6 although the mechanism for the preservation of pH to normal values in the 1st or 2nd stage of CKD is still being investigated.24 Acidosis should be confirmed by arterial blood gas analysis and the concentration of bicarbonate should be lower than 17 mEq/L, while the concentration of total CO2 should be lower than 15 mmol/L.2 Hypercholesterolaemia has been also found in cats with CRF, which has not developed secondary to glomerulonephritis.6,18 The potential increased activity in serum lipase and amylase may be due to their decreased elimination from the kidneys.14 According to the authors’ experience the constant biochemical abnormalities in cats with CRF, when present, are azotaemia and hyperphosphataemia.
Urinalysis is necessary for evaluation of the urinary system. In CKD, the reduced concentrating ability of the kidneys results in decreased urine specific gravity (USG) that is usually lower than 1035 and ranges between 1008 and 1015.2,4 Some cats may retain their ability to concentrate urine; thus, this finding should not mislead the clinician to the conclusion of prerenal or postrenal azotaemia. Proteinuria may be apparent, even in the absence of glomerulopathy, and has been related to shorter survival times26 or arterial hypertension.32 Minkus et al12 insists that when proteinuria is evident in cats with CRF, due to chronic tubulointerstitial nephritis, there is always concurrent glomerulonephritis. Proteinuria can be roughly assessed by sulfosalicylic acid precipitation test; however, its objective index is the urine protein to creatinine ratio, which should be greater than 0.4.3,4,22,23 In our clinic, proteinuria is a rather sporadic finding in cats with CRF. Microalbuminuria is an important diagnostic tool, which may contribute to diagnosing the inciting cause.4,7,22 CRF is not usually accompanied by an active sediment,4 unless it reflects the primary agent (e.g. pyelonephritis).14 Although no practical biomarker exists to reliably identify early CKD, determination of urine protein to creatinine ratio along with serum creatinine concentration have been suggested as potential predictors of the development of azotaemia in geriatric cats.33
In cases of secondary or primary bacterial infection of the lower urinary tract, urine culture should be performed. Pyelonephritis may result in CRF.7 Elderly cats are prone to urinary tract infections due to a disrupted immune system or CKD that limits local immunity. Finally, diluted urine loses its antibacterial properties.7,34 Low urine specific gravity may lead to negative results on urinalysis. In one study, 17 of the 77 cats with CKD had urinary tract infections, although only 4 of them showed clinical signs of lower urinary tract disease and a substantial number did not have WBCs or bacteria in their urine sediment.34
Radiology may contribute to the evaluation of kidney integrity and the definitive diagnosis. Radiography and ultrasonography of the upper and lower urinary system may rule out other possible causes of the cat’s signs. Moreover, upper urinary tract uroliths, hydronephrosis, polycystic kidney disease or kidney neoplasm may be detected. Computed tomography and intravenous urography may be used to evaluate GFR and confirm a definitive diagnosis.12,16
Evaluation of GFR should be used for the diagnosis of CKD, at least at the initial stages (1st or 2nd stage), where serum creatinine concentrations may be insensitive. Unfortunately, it does not provide the proportion of renal tissue affected by the kidney disease. A crude/poor estimation of GFR may be routinely made by determination of endogenous creatinine in serum. It is of note that serum creatinine value in the upper reference range still may reflect a decreased GFR. Exogenous administration of various substances that are eliminated mainly by the kidneys such as creatinine, inuline or iohexol is a better indicator of GFR. The keynote of this test is that after the administration of the substance, its concentration level in plasma or urine reflects its rate of elimination from the kidneys.4,20,35 Presumably, this test may be proven useful in estimating GFR also in advanced cases (3rd or 4th stage) where muscle wasting would contribute in lower serum creatinine concentration.
Systemic hypertension has been observed in 20% of cats with CKD,31 although percentages of 60- 65% have also been reported.36,37 The wide range may reflect the origin of the studies, suggesting there is variation between those coming from first and second opinion clinics.31 It is of interest that the incidence of arterial hypertension, secondary to CKD, in the cats we examined seemed low. However, determination of blood pressure is imperative due to the systemic, as well as renal consequences of hypertension.38 Moreover, arterial hypertension demands specific therapeutic management and defines the prognosis; although, if treated, it does not seem to affect survival time.32 As mentioned, ocular manifestation of hypertension must also be determined.31,38
Surgical or ultrasound guided renal biopsy and subsequent histopathology may result in a definitive diagnosis.7 It has been reported that in most cats (70.4%) chronic tubulointerstitial nephritis was detected histologically.12 Although histopathology is a rather insensitive method for the aetiological diagnosis of CKD, it is the cornerstone for the diagnosis of kidney disease before the establishment of renal dysfunction.20,25 In general, although biopsy of the kidneys is a relatively easy procedure in the cat, the risk of surgery does not outstand the benefits in the majority of feline patients.
Cats must also be tested for feline immunodeficiency virus (FIV), feline leukemia virus (FeLV), and feline infectious peritonitis (FIP) because CKD and its prognosis could be related to them.14 It has been recently reported that cats with CKD younger than 11 years old were more likely to be positive for FIV infection than were cats without CKD.39 A differentiation between acute and chronic disease should be made when uraemic crisis is apparent; it should be based on long lasting polyuria/polydipsia, small sized and irregular kidneys on palpation, and nonregenerative anaemia. A useful tip is that acute renal failure is characterized by quite intense clinical signs of relatively short duration.40
Heart examination, thyroid function estimation, ACTH stimulation test, and diabetes mellitus identification should be considered to determine prerenal causes of azotaemia or primary causes of CKD. Of note, the potential occurrence of both CKD and hyperthyroidism in aged cats may mask the magnitude of CRF because the hyperthyroid state improves renal blood supply and GFR.40
> Case presentation
Leosthenis, a 4-year-old male castrated Persian cat, was referred with a history of decreased appetite, depression, and sporadic episodes of vomiting. He exhibited five episodes of vomiting, consisting of gastric fluid, during the last 20 days. His owner did not notice any other signs, such as polyuria/polydipsia.
History Leosthenis was in good health throughout his life. He was an indoor cat, current on vaccination, routinely dewormed, and fed a commercial maintenance diet. Approximately three weeks before, he underwent a routine ultrasonic scaling of the teeth by the referring veterinarian. Physical examination, haematology, and biochemistry prior to the procedure were unremarkable, and recovery from anaesthesia was uneventful. However, Leosthenis showed signs of decreased appetite and depression, which were attributed to the unpleasant sensation of gingival irritation. When vomiting presented, gastroesophageal reflux and/or acute gastritis were suspected and ranitidine, metoclopramide, and sucralfate were prescribed. While the cat’s condition remained unchanged, CBC and biochemistry approximately 15 days later revealed marked azotaemia.
Physical and laboratory evaluation Leosthenis was mildly dehydrated and depressed on presentation. PCV was in the lower normal values (25.3%; ref. range: 24-45%), while BUN (160 mg/ dl; ref. range: 9-32 mg/dl), creatinine (4.1 mg/dl; ref. range: 0.7- 1.6 mg/dl), and phosphorus (8.9 mg/dl; ref. range: 3.5-6.7 mg/dl) were markedly increased. Potassium (2.2 mEq/L; ref. range: 3.4-5.4 mEq/L) was decreased, USG was 1017, and sediment examination did not reveal any pathological findings.
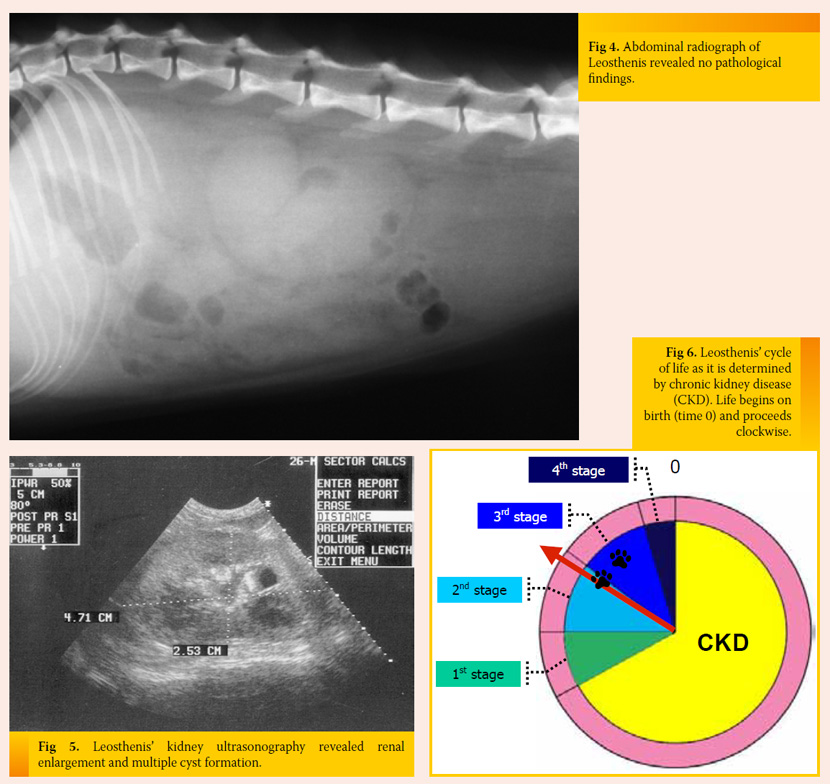
Initial measures and further diagnostic work-up IV fluids supplemented with potassium and ranitidine were initially provided. Abdominal radiography showed the size of the kidneys (especially the right kidney) was in the upper limits (Fig 4), and ultrasound revealed multiple cysts and enlarged size of both kidneys (Fig 5). UPC was normal and urine culture was negative. Moreover, arterial blood pressure was within normal limits.
Diagnosis Polycystic kidney disease in a Persian cat was the definitive diagnosis in this case. Anaesthesia may have provoked the CKD to be symptomatic.
Treatment and outcome Leosthenis was released on the 6th day of hospitalization. He was happy, active, and eating sufficiently, but he was mildly azotaemic, hyperphosphataemic, and normokalaemic. A prescription diet for feline kidney disease, ranitidine, a phosphate binder, and an ACE inhibitor were administered. Serial biochemistry showed stabilization of Leosthenis’ condition and he was currently in the 2nd stage of CKD, which remained unchanged until now, one year later (Fig 6).
What would this case suggest? It is well known that there is a familial predisposition of polycystic kidney disease in Persian cats. Most of these cats exhibit CRF about 7 years of age, although some may remain clinically normal for most of their lives. Remission of the disease may be also evident.45 Subsequently, susceptible cats should be handled with caution when invasive procedures are needed to preserve their kidney function and integrity. Early recognition may be crucial. Ultrasound examination is recommended as early as 10 months of age.45 Genetic testing, if available, is reliable diagnostic method, but it does not determine the severity of the disease.
> Consequences of chronic renal failure
In cats with CRF, uraemic toxins accumulate in organic fluids due to dysregulation of the kidney’s capacity to excrete metabolic waste products. These organic compounds are responsible for the variable and polysystematic consequences or complications of the disease. Furthermore, the complications of CRF result from hormonal disturbances and the inability of the kidneys to regulate body fluids, as well acid-base and electrolyte homeostasis.5
1. Polyuria and polydipsia
Polyuria is induced by a reduction in the urine concentrating ability of the kidneys, which is the result of diminished responsiveness of the kidneys to antidiuretic hormone, disruption of the renal medullary architecture, and solute diuresis.40 A compensation of polyuria is polydipsia, which seems to be recognised more commonly from polyuria by cat owners.
2. Arterial hypertension
When both systemic arterial hypertension and CKD present, cause and effect determination is difficult.38 Primary hypertension is very common in humans; while secondary arterial hypertension is reported to occur in up to 66% of cats with CKD. Although characteristic histopathological lesions of hypertension have been found in the kidneys of dogs and cats with CRF, the hypertensive nephropathy seen in humans is not thought to exist in small animals. These lesions are believed to be the result of hypertension occurring secondary to CKD.2 In general, arterial pressure seems to be regulated by adrenergic, renal, endocrine and vascular systems and it is related to cardiac output and vascular resistance. In animals with CRF, dysregulation of fluid homeostasis due to decreased GFR and reduced sodium excretion, increase of cardiac output, disturbance of vessel resistance, and impairment of reninangiotensin- aldosterone as well kallikrein-kininprostaglandin systems and effects related to hyperparathyroidism have been incriminated for the development of hypertension.17,38 It should be mentioned that hyperaldosteronism has led to hypertension in some cats with CRF.41
3. Renal secondary hyperparathyroidism
Renal secondary hyperparathyroidism (RSH) is one of the most common and significant consequences of CRF.42 It is characterized by increased activity of parathormone (PTH) in serum and has been observed in about 80% of cats with CKD.40 The increase of PTH results from reduced production of calcitriol in renal tubular cells and the retention of phosphorus from the kidneys.23 Phosphorus retention leads to hyperphosphataemia, which in turn results in cessation of the conversion of 25-hydroxycholocalciferol to the most active form of vitamin D, calcitriol, by 1a-hydroxylase. The reduced concentration of calcitriol and, subsequently, calcium in serum stimulates PTH production from parathyroid glands.2 PTH is considered one of the main uraemic toxins;5 although rare in cats, signs of RSH are observed when serum PTH concentration increases resulting in skeletal release of calcium and bone demineralization (renal osteodystrophy) and subsequent soft tissue calcification,23,42 as well various organs malfunction. Cellular dysfunction was observed when persistently increased basal cytosolic calcium levels were present. In humans, bones of the skull and mandible are most severely affected and the sensation of generalized bone pain may be evident.43 Increased PTH levels lead to adverse reactions to glucose and lipid metabolism, as well to cardiovascular and CNS functions. Moreover, it deteriorates immunodeficiency and anaemia, which are already complications of feline CRF itself.44 As it has been already mentioned, laboratory findings of RSH include increased concentration of serum phosphorus and PTH. Increased risk of calcification exists when the product of multiplication of the concentrations of serum phosphorus times the total serum calcium is greater than 70.23 Proton-secreting organs, like stomach and kidneys are most commonly affected, but also myocardium, lung and liver can be mineralized. It is of note that in several cats with CRF, hyperparathormonaemia was apparent despite the absence of hyperphosphataemia.40,43 Parathyroidomegaly secondary to RSH may be observed as a palpable paratracheal mass and should not be confused with a thyroid nodule due to hyperthyroidism, which is a frequent coincident to CKD.
4. Gastrointestinal disturbances
Gastrointestinal complications, namely various combinations of anorexia, weight loss and haemorrhagic or not vomiting and diarrhoea are the signs that may observed in cats especially with advanced CRF (3rd or 4th stage). The stomach is most commonly affected, but signs from the rest of the gastrointestinal tract may be seen. Ulcerative gastritis may result from hypergastrinaemia, which is a consequence of decreased GFR. Increased gastrin levels may cause hyperacidity due to increased production of hydrogen ions from the gastric parietal cells. Also, disease stress, gastric mucosa ischemia, and impaired gastric mucus are some additional factors that may contribute to ulcerative gastritis. Finally, ammonia and urea directly result in dryness, hyperaemia, erosions, and ulcers of the gastrointestinal mucosa and may lead to ulcerative stomatitis, oesophagitis, gastritis, and enterocolitis.23
5. Anaemia
Cats with CKD usually present with nonregenerative, normochromic, and normocytic anaemia which may be multifactorial.40 The major cause of anaemia is erythropoietin deficiency due to insufficient capacity of peritubular fibroblasts of the renal cortex and/or interstitial fibroblasts to synthesize erythropoietin.6 It is of note that plasma erythropoietin levels of anaemic CKD cats are lower than those of anaemic non-CKD cats, while they are equal to those of healthy cats. Chronic blood loss from the gastrointestinal tract, malnutrition, hypoproteinaemia, weight loss, vitamin or other nutrient deficiency, shortened red cell life span, erythropoietic inhibitors in uraemic plasma, myelofibrosis, haemorrhagic diathesis due to platelet dysfunction, and effects from chronic disease are additional factors contributing to CKD related anaemia in cats.2,40
6. Acid – base and electrolyte disturbance
Hypokalaemia is one of the most important electrolyte disturbances in cats with CRF.44 Hypokalaemia is the result of reduced potassium intake, potassium loss through the kidneys, acidbase disturbance, and reduced serum magnesium concentration,25,31 and it may be developed long after the onset of decreased muscle potassium content. Restoration of normokalaemia has been considered mandatory, due to GFR malfunction caused by potassium depletion. As previously mentioned, hyperphosphataemia is the most common electrolyte abnormality in cats with azotaemic CRF and is reported in up to 80% of cases.2 Based on the authors’ experience, the principal electrolyte disorder in azotaemic cats with CKD is hyperphosphataemia.
 The vast majority of the CRF cats with acidbase imbalance show metabolic acidosis, which results from reduced bicarbonate reabsorption, impaired renal ammoniagenesis, and decreased renal excretion of hydrogen ions.2,44 In a small number of cases metabolic alkalosis may be evident. In a retrospective study in cats with CRF, metabolic acidosis was seen in 50% of cats with end stage disease, in 15% with moderate disease, and was rarely evident in cases in the 1st or 2nd stage. In acute uraemic crisis, acidaemia could cause decrease in central and pulmonary vascular compliance, which may result in increased risk of pulmonary oedema development. Metabolic acidosis of long duration increases protein degradation, deteriorates renal osteodystrophy, and results in further renal damage.24
The vast majority of the CRF cats with acidbase imbalance show metabolic acidosis, which results from reduced bicarbonate reabsorption, impaired renal ammoniagenesis, and decreased renal excretion of hydrogen ions.2,44 In a small number of cases metabolic alkalosis may be evident. In a retrospective study in cats with CRF, metabolic acidosis was seen in 50% of cats with end stage disease, in 15% with moderate disease, and was rarely evident in cases in the 1st or 2nd stage. In acute uraemic crisis, acidaemia could cause decrease in central and pulmonary vascular compliance, which may result in increased risk of pulmonary oedema development. Metabolic acidosis of long duration increases protein degradation, deteriorates renal osteodystrophy, and results in further renal damage.24
> Conclusion
Feline CKD is a very common disorder and predominates in elderly cats. In most cases, the aetiology remains unclear. The diagnostic approach involves evaluation of the cause of CKD, its severity (stage), and its effects on the kidneys and other organs or systems. Moreover, the diagnosis aims to pursue the appropriate measures and establish the prognosis of the disease.
> References
1. Lawler DF, Evans RH, Chase K, Ellersieck M, Li Q, Larson BT, Satyarai E, Heininqer K. The aging feline kidney: a model mortality antagonist? J Feline Med Surg 2006, 8: 363-371.
2. Ross SJ, Polzin DJ, Osborne CA. Clinical progression of early chronic renal failure and implications for management. In: Consultations in Feline Internal Medicine. August JR (ed). Vol 5. Elsevier Saunders: St Louis, 2006, pp. 389-398.
3. White JD, Malik R, Norris JM. Feline chronic kidney disease: can we move from treatment to prevention? Vet J 2011, doi: 10.1016/j.tvjl.2010.12.011.
4. Lees GE. Early diagnosis of renal disease and renal failure. Vet Clin Small Anim Pract 2004, 34: 867-885.
5. Glassock RJ. Uremic toxins: what are they? An integrated overview of pathobiology and classification. J Ren Nutr 2008, 18: 2-6.
6. Elliott J, Barber PJ. Feline chronic renal failure: clinical findings in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract 1998, 39: 78-85.
7. Grauer GF. Early detection of renal damage and disease in dogs and cats. Vet Clin Small Anim Pract 2005, 35: 581-596.
8. Sturgess K. Renal disease. In: Notes in Feline Internal Medicine. Blackwell Science Ltd: Oxford, 2003, pp. 147-165.
9. Cannon MJ, MacKay AD, Barr FJ, Rudorf H, Bradley KJ, Gruffydd-Jones TJ. Prevalence of polycystic kidney disease in Persian cats in the United Kingdom. Vet Rec 2001, 149: 409-411.
10. Barthez PY, Rivier P, Begon D. Prevalence of polycystic kidney disease in Persian and Persian related cats in France. J Feline Med Surg 2003, 5: 345-347.
11. Ross SJ, Osborne CA, Lekcharoensuk C, Koehler LA, Polzin DJ. A case-control study of the effects of nephrolithiasis in cats with chronic kidney disease. J Am Vet Med Assoc 2007, 230: 1854-1859.
12. Minkus G, Reusch C, Hörauf A, Breuer W, Darbès J, Kraft W, Hermanns W. Evaluation of renal biopsies in cats and dogs-histopathology in comparison with clinical data. J Small Anim Pract 1994, 35: 465-472.
13. Lappin MR, Jensen WA, Jensen TD, Basaraba RJ, Brown CA, Radecki SV, Hawley JR. Investigation of the induction of antibodies against Crandell-Rees feline kidney cell lysates and feline renal cell lysates after parenteral administration of vaccines against feline viral rhinotracheitis, calicivirus and panleukopenia in cats. Am J Vet Res 2005, 66: 506-511.
14. Polzin DJ, Osborne CA, Ross S. Chronic kidney disease. In: Textbook of Veterinary Medicine. Diseases of the Dog and Cat. Ettinger SJ, Feldman EC (eds). 6th edn. Vol 2. Elsevier Saunders: St Louis, 2005, pp. 1756-1785.
15. German AC, Harbour DA, Helps CR, Gruffydd-Jones TJ. Is feline foamy virus really apathogenic? Vet Immunol Immunopathol 2008, 123: 114-118.
16. Kyles AE, Hardie EM, Wooden BG, Adin CA, Stone EA, Gregory CR, Mathews KG, Cowgill LD, Vaden S, Nyland TG, Ling GV. Clinical, clinicopathologic, radiographic, and ultrasonographic abnormalities in cats with ureteral calculi: 163 cases (1984-2002). J Am Vet Med Assoc 2005, 226: 932-936.
17. Watanabe T, Mishina M. Effects of benazepril hydrochloride in cats with experimentally induced or spontaneously occurring chronic renal failure. J Vet Med Sci 2007, 69: 1015-1023.
18. DiBartola SP, Rutgers HC, Zack PM, Tarr MJ. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973-1984). J Am Vet Med Assoc 1987, 190: 1196-1202.
19. Ross SJ, Osborne CA, Kirk CA, Lowry SR, Koehler LA, Polzin DJ. Clinical evaluation of dietary modification for treatment of spontaneous chronic kidney disease in cats. J Am Vet Med Assoc 2006, 229: 949-957.
20. Finco DR, Brown SA, Brown CA, Crowell WA, Cooper TA, Barsanti JA. Progression of chronic renal disease in the dog. J Vet Intern Med 1999, 13: 516-528.
21. Brown SA, Brown CA, Jacobs G, Stiles J, Hendi RS, Wilson S. Effects of the angiotensin converting enzyme inhibitor benazepril in cats with induced renal insufficiency. Am J Vet Res 2001, 62: 375-383.
22. Syme H. Proteinuria in cats. Prognostic marker or mediator? J Feline Med Surg 2009, 11: 211-218.
23. Plotnick A. Feline chronic renal failure: long-term medical management. Compend Contin Educ Pract Vet 2007, 29: 342-351.
24. Elliott J, Syme HM, Markwell PJ. Acid-base balance of cats with chronic renal failure: effect of deterioration in renal function. J Small Anim Pract 2003, 44: 261-268.
25. Adams LG, Polzin DJ, Osborne CA, O’Brien TD. Effects of dietary protein and calorie restriction in clinically normal cats and in cats with surgically chronic renal failure. Am J Vet Res 1993, 54: 1653-1662.
26. King JN, Tasker S, Gunn-Moore DA, Strehlau G, BENRIC (Benazepril in renal insufficiency in cats) Study Group. Prognostic factors in cats with chronic kidney disease. J Vet Intern Med 2007, 21: 906-916.
27. Lund EM, Armostrong PJ, Kirk CA, Kolar LM, Klausner JS. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J Am Vet Med Assoc 1999, 214: 1336-1341.
28. Watson A. Indicators of renal insufficiency in dogs and cats presented at a veterinary teaching hospital. Austral Vet Pract 2001, 31: 54-58.
29. White JD, Norris JM, Baral RM, Malik R. Naturally – occurring chronic renal disease in Australian cats: a prospective study of 184 cases. Austr Vet J 2006, 84: 188-194.
30. Heiene R, Rumsby G, Ziener M, Dahl SA, Tims C, Teiqe J, Ottesen N. Chronic kidney disease with three cases of oxalate-like nephrosis in Ragdoll cats. J Feline Med Surg 2009, 11: 474-480.
31. Syme HM, Barber PJ, Markwell PJ, Elliott J. Prevalence of systolic hypertension in cats with chronic renal failure at initial evaluation. J Am Vet Med Assoc 2002, 220: 1799-1804.
32. Syme HM, Markwell PJ, Pfeiffer D, Elliott J. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J Vet Intern Med 2006, 20: 528-535.
33. Jepson RE, Brodbelt D, Vallance C, Syme HM, Elliott J. Evaluation of predictors of the development of azotemia in cats. J Vet Intern Med 2009, 23: 806-813.
34. Mayer-Roenne B, Goldstein RE, Erb HN. Urinary tract infections in cats with hyperthyroidism, diabetes mellitus and chronic kidney disease. J Feline Med Surg 2007, 9: 124-132.
35. Miyagawa Y, Takemura N, Hirose H. Assessments of factors that affect glomerular filtration rate and internal markers of renal function in dogs and cats. J Vet Med Sci 2010, 72: 1129-1136.
36. Kobayashi DL, Peterson ME, Graves TK, Lesser M, Nichols CE. Hypertension in cats with chronic renal failure or hyperthyroidism. J Vet Intern Med 1990, 4: 58- 62.
37. Stiles J, Polzin DJ, Bistner SI. The prevalence of retinopathy in cats with systemic hypertension and chronic renal failure or hyperthyroidism. J Am Anim Hosp Assoc 1994, 30: 564-572.
38. Jepson R. Feline systemic hypertension. Classification and pathogenesis. J Feline Med Surg 2011, 13: 25-34.
39. White JD, Malik R, Norris JM, Malkides N. Association between naturally occurring chronic kidney disease and feline immunodeficiency virus infection status in cats. J Am Vet Med Assoc 2010, 236: 424-429.
40. Barber P, Miller JB, Rand J. The thin, inappetent cat. In: Problem-Based Feline Medicine. Rand J (ed). Elsevier Saunders: Edinburgh, 2006, pp. 330-363.
41. Jensen J, Henik RA, Brownfield M, Armstrong J. Plasma renin activity and angiotensin I and aldosterone consentrations in cats with hypertension associated with chronic renal disease. Am J Vet Res 1997, 58: 535- 540.
42. Elliott J, Rawlings JM, Markwell PJ, Barber PJ. Survival of cats with naturally occurring chronic renal failure: effect of dietary management. J Small Anim Pract 2000, 41: 235-242.
43. Barber PJ, Rawlings JM, Markwell PJ, Elliott J. Effect of dietary phosphate restriction on renal secondary hyperparathyroidism in the cat. J Small Anim Pract 1999, 40: 62-70.
44. Polzin DJ, Osborne CA, Ross S, Jacob F. Dietary management of feline chronic renal failure: where are we now? In what direction are we headed? J Feline Med Surg 2000, 2: 75-82.
45. Wills SJ, Barrett EL, Barr FJ, Bradley KJ, Helps CR, Cannon MJ, Gruffydd-Jones TJ. Evaluation of the repeatability of ultrasound scanning for detection of feline polycystic kidney disease. J Feline Med Surg 2009, 11: 993-996.



